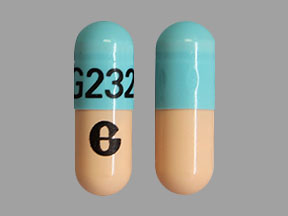
Omeprazole
Pronunciation: oh-MEP-ra-zol
Generic name: omeprazole
Brand names: FIRST Omeprazole, Omeprazole + SyrSpend SF Alka, PriLOSEC, PriLOSEC OTC, Zegerid (Original Formulation)
Dosage forms: oral delayed release capsule (10 mg; 20 mg; 40 mg), oral delayed release tablet (20 mg),
... show all 4 dosage forms
Drug class: Proton pump inhibitors
What is omeprazole?
Omeprazole is used to treat excess stomach acid in conditions such as non cancerous stomach ulcers, gastroesophageal reflux disease (GERD), active duodenal ulcer, Zollinger-Ellison syndrome and erosive esophagitis. Omeprazole works by blocking gastric acid production and is from the group of medicines called proton pump inhibitors.
Omeprazole may also be given together with antibiotics to treat gastric ulcer caused by infection with Helicobacter pylori (H. pylori).
Over-the-counter (OTC) omeprazole is used in adults to help control heartburn that occurs 2 or more days per week. The OTC brand must be taken as a course on a regular basis for 14 days in a row.
Warnings
Omeprazole can cause kidney problems. Tell your doctor if you are urinating less than usual, or if you have blood in your urine.
Diarrhea may be a sign of a new infection. Call your doctor if you have diarrhea that is watery or has blood in it.
Omeprazole is not to used for the immediate relief of heartburn symptoms.
Omeprazole may cause new or worsening symptoms of lupus. Tell your doctor if you have joint pain and a skin rash on your cheeks or arms that worsens in sunlight.
You may be more likely to have a broken bone while taking this medicine long term or more than once per day.
Before taking this medicine
Heartburn can mimic early symptoms of a heart attack. Get emergency medical help if you have chest pain that spreads to your jaw or shoulder and you feel sweaty or light-headed.
You should not use omeprazole if you are allergic to it, or if:
-
you are also allergic to medicines like omeprazole, such as esomeprazole, lansoprazole, pantoprazole, rabeprazole, Nexium, Prevacid, Protonix, and others;
-
you had breathing problems, kidney problems, or a severe allergic reaction after taking omeprazole in the past; or
-
you also take HIV medication that contains rilpivirine (such as Complera, Edurant, Odefsey, Juluca).
Ask a doctor or pharmacist if this medicine is safe to use if you have:
-
trouble or pain with swallowing;
-
bloody or black stools, vomit that looks like blood or coffee grounds;
-
heartburn that has lasted for over 3 months;
-
frequent chest pain, heartburn with wheezing;
-
unexplained weight loss;
-
liver disease;
-
low levels of magnesium in your blood; or
-
osteoporosis or low bone mineral density (osteopenia).
You may be more likely to have a broken bone in your hip, wrist, or spine while taking a proton pump inhibitor long-term or more than once per day. Talk with your doctor about ways to keep your bones healthy.
Ask a doctor before using this medicine if you are pregnant or breastfeeding.
Do not give this medicine to a child without medical advice.
How should I take omeprazole?
Take omeprazole exactly as directed on the label, or as prescribed by your doctor. Follow all directions on your prescription label and read all medication guides or instruction sheets.
Use Prilosec OTC (over-the-counter) exactly as directed on the label, or as prescribed by your doctor.
Read and carefully follow any Instructions for Use provided with your medicine. Ask your doctor or pharmacist if you do not understand these instructions.
Shake the oral suspension (liquid) before you measure a dose. Use the dosing syringe provided, or use a medicine dose-measuring device (not a kitchen spoon).
If you cannot swallow a capsule whole, open it and sprinkle the medicine into a spoonful of applesauce. Swallow the mixture right away without chewing. Do not save it for later use.
You must dissolve omeprazole powder in a small amount of water. This mixture can either be swallowed or given through a nasogastric (NG) feeding tube using a catheter-tipped syringe.
Use this medicine for the full prescribed length of time, even if your symptoms quickly improve.
OTC omeprazole should be taken for only 14 days in a row. It may take 1 to 4 days before your symptoms improve. Allow at least 4 months to pass before you start a new 14-day course of treatment.
Call your doctor if your symptoms do not improve, or if they get worse.
Some conditions are treated with a combination of omeprazole and antibiotics. Use all medications as directed.
This medicine can affect the results of certain medical tests. Tell any doctor who treats you that you are using this medicine.
Store at room temperature away from moisture and heat.
What happens if I miss a dose?
Take the medicine as soon as you can, but skip the missed dose if it is almost time for your next dose. Do not take two doses at one time.
What happens if I overdose?
Seek emergency medical attention or call the Poison Help line at 1-800-222-1222.
What to avoid
Omeprazole can cause diarrhea, which may be a sign of a new infection. If you have diarrhea that is watery or bloody, call your doctor before using anti-diarrhea medicine.
Omeprazole side effects
Get emergency medical help if you have signs of an allergic reaction to omeprazole: hives; difficulty breathing; swelling of your face, lips, tongue, or throat.
Stop using this medicine and call your doctor at once if you have:
-
severe stomach pain, diarrhea that is watery or bloody;
-
new or unusual pain in your wrist, thigh, hip, or back;
-
seizure (convulsions);
-
kidney problems - fever, rash, nausea, loss of appetite, joint pain, urinating less than usual, blood in your urine, weight gain;
-
low magnesium - dizziness, irregular heartbeats, feeling jittery, muscle cramps, muscle spasms, cough or choking feeling; or
-
new or worsening symptoms of lupus - joint pain, and a skin rash on your cheeks or arms that worsens in sunlight.
Taking omeprazole long-term may cause you to develop stomach growths called fundic gland polyps. Talk with your doctor about this risk.
If you use this medicine for longer than 3 years, you could develop a vitamin B-12 deficiency. Talk to your doctor about how to manage this condition if you develop it.
Common side effects of omeprazole may include:
-
cold symptoms such as stuffy nose, sneezing, sore throat (especially in children);
-
fever (especially in children);
-
stomach pain, gas;
-
nausea, vomiting, diarrhea; or
This is not a complete list of side effects and others may occur. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Related/similar drugs
What other drugs will affect omeprazole?
Sometimes it is not safe to use certain medications at the same time. Some drugs can affect your blood levels of other drugs you take, which may increase side effects or make the medications less effective.
Tell your doctor about all your current medicines. Many drugs can affect omeprazole, especially:
-
a diuretic or "water pill"; or
-
an antibiotic - amoxicillin, clarithromycin, rifampin.
This list is not complete and many Other drugs may interact with omeprazole. This includes prescription and over-the-counter medicines, vitamins, and herbal products. Not all possible drug interactions are listed here.
Popular FAQ
Pantoprazole vs. omeprazole: What's the difference between them?
Pantoprazole and omeprazole are both medicines from the class of medications called proton pump inhibitors (PPIs). The approved uses for pantoprazole and omeprazole differ slightly, but they are all disorders related to too much stomach acid. Continue reading
Can you take an antacid with omeprazole?
You can take an antacid with omeprazole if you are still getting symptoms of indigestion because it can take several days for omeprazole to start working. Take omeprazole as directed (usually taken once daily on an empty stomach), and take antacids as needed to relieve indigestion pain after eating. But tell your doctor if several weeks have gone past and omeprazole does not seem to be working or you are still taking antacids. Continue reading
Is famotidine safer than omeprazole for heartburn?
Famotidine is usually the first choice to treat occasional heartburn if your symptoms occur less than two times per week. It can be used as needed, provides quick relief (within 15 to 30 minutes), and is usually less expensive than omeprazole. Omeprazole may be an option if your heartburn occurs two or more days per week. Both products are available without a prescription. Continue reading
Can I take omeprazole in the morning and famotidine at night?
Yes, you could take omeprazole in the morning and famotidine at night but there are not many studies investigating this approach. Preliminary results suggest that taking your medication this way may provide superior control of gastric acid secretion at night without compromising the effectiveness of omeprazole in the morning. More research is needed. Continue reading
Is omeprazole (Prilosec) bad for your kidneys?
Omeprazole (Prilosec) is a proton pump inhibitor (PPI) that is used to reduce the amount of acid in your stomach and it can be bad for your kidneys. It can cause acute kidney injury and may also worsen the progression of chronic kidney disease (CKD). Continue reading
Does omeprazole cause cancer?
Long-term use of proton pump inhibitor (PPI) acid-suppressing agents, such as omeprazole, has been associated with an increased risk of stomach cancer (also called gastric cancer). This is based on the results of several observational studies and is thought to be because PPIs such as omeprazole are potent gastric acid suppressants that may increase the risk of gastric cancer by causing atrophy (thinning) of the stomach lining, elevating levels of a hormone called gastrin, and an overgrowth of bacteria in the stomach. Continue reading
More FAQ
- What foods should I avoid when taking omeprazole?
- How soon after taking levothyroxine can I take omeprazole?
- Should I take omeprazole with or without food?
More about omeprazole
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (502)
- Drug images
- Side effects
- Dosage information
- Patient tips
- During pregnancy
- Support group
- Drug class: proton pump inhibitors
- Breastfeeding
Patient resources
Other brands
Professional resources
- Omeprazole monograph
- Omeprazole (FDA)
- Omeprazole Magnesium Granules (FDA)
- Omeprazole ODT (FDA)
- Omeprazole Tablets (FDA)
- TopCare Omeprazole (FDA)
Other brands
Related treatment guides
Further information
Remember, keep this and all other medicines out of the reach of children, never share your medicines with others, and use omeprazole only for the indication prescribed.
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
Copyright 1996-2025 Cerner Multum, Inc. Version: 21.02.