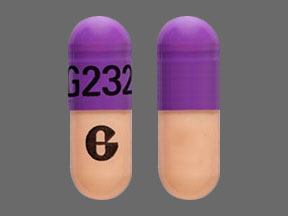
G G232 Pill: blue & orange, capsule/oblong, 19mm
The pill with imprint G G232 (Blue & Orange, Capsule/Oblong, 19mm) has been identified as Omeprazole Delayed-Release 40 mg and is used for Barrett's Esophagus, Erosive Esophagitis, Duodenal Ulcer, GERD, and Indigestion. It belongs to the drug class proton pump inhibitors and is not a controlled substance.
Images for G G232
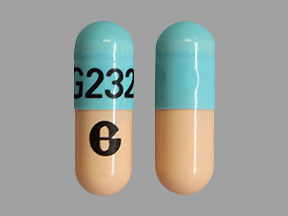
Omeprazole Delayed-Release
- Imprint
- G G232
- Strength
- 40 mg
- Color
- Blue & Orange
- Size
- 19.00 mm
- Shape
- Capsule/Oblong
- Availability
- Rx and/or OTC
- Drug Class
- Proton pump inhibitors
- Pregnancy Category
- C - Risk cannot be ruled out
- CSA Schedule
- Not a controlled drug
- Labeler / Supplier
- Glenmark Pharmaceuticals Inc., USA
- National Drug Code (NDC)
- 68462-0232 (Discontinued)
Related images for "G G232"
More about omeprazole
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (502)
- Drug images
- Side effects
- Dosage information
- Patient tips
- During pregnancy
- Support group
- Drug class: proton pump inhibitors
- Breastfeeding
Patient resources
Other brands
Professional resources
- Omeprazole monograph
- Omeprazole (FDA)
- Omeprazole Magnesium Granules (FDA)
- Omeprazole ODT (FDA)
- Omeprazole Tablets (FDA)
- TopCare Omeprazole (FDA)
Other brands
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.