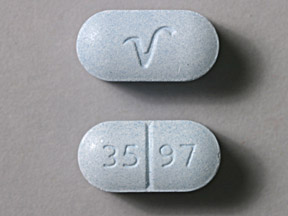
35 97 V Pill: blue, capsule/oblong, 16mm
The pill with imprint 35 97 V (Blue, Capsule/Oblong, 16mm) has been identified as Acetaminophen and Hydrocodone Bitartrate 650 mg / 10 mg and is used for Back Pain, Pain, and Cough. It belongs to the drug class narcotic analgesic combinations and is classified as CSA Schedule 2 (High potential for abuse).
Images for 35 97 V

Acetaminophen and Hydrocodone Bitartrate
- Imprint
- 35 97 V
- Strength
- 650 mg / 10 mg
- Color
- Blue
- Size
- 16.00 mm
- Shape
- Capsule/Oblong
- Availability
- Prescription only
- Drug Class
- Narcotic analgesic combinations
- Pregnancy Category
- C - Risk cannot be ruled out
- CSA Schedule
- 2 - High potential for abuse
- Labeler / Supplier
- Qualitest Pharmaceuticals Inc.
- National Drug Code (NDC)
- 00603-3885 (Discontinued)
- Inactive Ingredients
-
silicon dioxide,
croscarmellose sodium,
FD&C Blue No. 1,
magnesium stearate,
microcrystalline cellulose,
povidone,
corn starch,
stearic acid,
crospovidone
Note: Inactive ingredients may vary.
See also:
More about acetaminophen / hydrocodone
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (1,318)
- Drug images
- Side effects
- Dosage information
- Patient tips
- During pregnancy
- Support group
- Drug class: narcotic analgesic combinations
- En español
Patient resources
Other brands
Norco, Vicodin, Lortab, Lorcet, ... +9 more
Professional resources
- Hydrocodone Bitartrate and Acetaminophen Oral Solution prescribing information
- Hydrocodone and Acetaminophen (FDA)
- Hydrocodone and Acetaminophen Capsules (FDA)
- Hydrocodone and Acetaminophen Elixir (FDA)
- Hydrocodone and Acetaminophen Oral Solution (FDA)
Other brands
Norco, Vicodin, Lortab, Hycet, ... +8 more
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
