Zamicet: Package Insert / Prescribing Info
Package insert / product label
Generic name: hydrocodone bitartrate and acetaminophen
Dosage form: oral solution
Drug class: Narcotic analgesic combinations
Medically reviewed by Drugs.com. Last updated on Mar 25, 2025.
The Zamicet brand name has been discontinued in the U.S. If generic versions of this product have been approved by the FDA, there may be generic equivalents available.
On This Page
Hepatotoxicity
Acetaminophen has been associated with cases of acute liver failure, at times resulting in liver transplant and death. Most of the cases of liver injury are associated with the use of acetaminophen at doses that exceed 4000 milligrams per day, and often involve more than one acetaminophen-containing product.
Zamicet Description
Zamicet® (hydrocodone bitartrate and acetaminophen oral solution) is supplied in liquid form for oral administration.
Warning
May be habit forming (see PRECAUTIONS, Information for Patients, and DRUG ABUSE AND DEPENDENCE).
Hydrocodone bitartrate is an opioid analgesic and antitussive which occurs as fine, white crystals or as a crystalline powder. It is affected by light. The chemical name is 4, 5α-epoxy-3-methoxy-17-methylmorphinan-6-one tartrate (1:1) hydrate (2:5). It has the following structural formula:
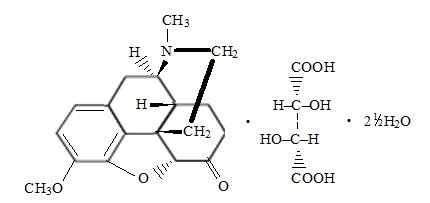
C18H21NO3∙ C4H6O6∙ 2½ H2O M.W. 494.490
Acetaminophen, 4'-hydroxyacetanilide, a slightly bitter, white, odorless, crystalline powder, is a non-opiate, non-salicylate analgesic and antipyretic. It has the following structural formula:
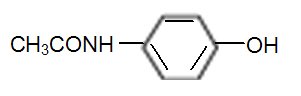
C8H9NO2 M.W. 151.16
Zamicet® contains:
| Per 7.5 mL | Per 15 mL | |
|---|---|---|
| Hydrocodone bitartrate | 5 mg | 10 mg |
| Acetaminophen | 163 mg | 325 mg |
| Alcohol | 6.7% | 6.7% |
In addition Zamicet® contains the following inactive ingredients: citric acid, edetate disodium, glycerin, methylparaben, propylene glycol, purified water, saccharin sodium, sorbitol solution, sucrose, with D&C Yellow No. 10 as coloring and natural and artificial flavoring.
Zamicet - Clinical Pharmacology
Hydrocodone is a semisynthetic narcotic analgesic and antitussive with multiple actions qualitatively similar to those of codeine. Most of these involve the central nervous system and smooth muscle. The precise mechanism of action of hydrocodone and other opiates is not known, although it is believed to relate to the existence of opiate receptors in the central nervous system. In addition to analgesia, narcotics may produce drowsiness, changes in mood and mental clouding.
The analgesic action of acetaminophen involves peripheral influences, but the specific mechanism is as yet undetermined. Antipyretic activity is mediated through hypothalamic heat regulating centers. Acetaminophen inhibits prostaglandin synthetase. Therapeutic doses of acetaminophen have negligible effects on the cardiovascular or respiratory systems; however, toxic doses may cause circulatory failure and rapid, shallow breathing.
Pharmacokinetics
The behavior of the individual components is described below.
Hydrocodone
Following a 10 mg oral dose of hydrocodone administered to five adult male subjects, the mean peak concentration was 23.6 ± 5.2 ng/mL. Maximum serum levels were achieved at 1.3 ± 0.3 hours and the half-life was determined to be 3.8 ± 0.3 hours. Hydrocodone exhibits a complex pattern of metabolism including O-demethylation, N-demethylation and 6-keto reduction to the corresponding 6-α- and 6-β-hydroxymetabolites.
See OVERDOSAGE for toxicity information.
Acetaminophen
Acetaminophen is rapidly absorbed from the gastrointestinal tract and is distributed throughout most body tissues. The plasma half-life is 1.25 to 3 hours, but may be increased by liver damage and following overdosage. Elimination of acetaminophen is principally by liver metabolism (conjugation) and subsequent renal excretion of metabolites. Approximately 85% of an oral dose appears in the urine within 24 hours of administration, most as the glucuronide conjugate, with small amounts of other conjugates and unchanged drug.
See OVERDOSAGE for toxicity information.
Indications and Usage for Zamicet
Zamicet® (hydrocodone bitartrate and acetaminophen oral solution) is indicated for the relief of moderate to moderately severe pain.
Contraindications
This product should not be administered to patients who have previously exhibited hypersensitivity to hydrocodone, acetaminophen, or any other component of this product.
Patients known to be hypersensitive to other opioids may exhibit cross-sensitivity to hydrocodone.
Warnings
Hepatotoxicity
Acetaminophen has been associated with cases of acute liver failure, at times resulting in liver transplant and death. Most of the cases of liver injury are associated with the use of acetaminophen at doses that exceed 4000 milligrams per day, and often involve more than one acetaminophen-containing product. The excessive intake of acetaminophen may be intentional to cause self-harm or unintentional as patients attempt to obtain more pain relief or unknowingly take other acetaminophen-containing products.
The risk of acute liver failure is higher in individuals with underlying liver disease and in individuals who ingest alcohol while taking acetaminophen.
Instruct patients to look for acetaminophen or APAP on package labels and not to use more than one product that contains acetaminophen. Instruct patients to seek medical attention immediately upon ingestion of more than 4000 milligrams of acetaminophen per day, even if they feel well.
Serious Skin Reactions
Rarely, acetaminophen may cause serious skin reactions such as acute generalized exanthematous pustulosis (AGEP), Stevens-Johnson Syndrome (SJS), and toxic epidermal necrolysis (TEN), which can be fatal. Patients should be informed about the signs of serious skin reactions, and use of the drug should be discontinued at the first appearance of skin rash or any other sign of hypersensitivity.
Hypersensitivity/Anaphylaxis
There have been post-marketing reports of hypersensitivity and anaphylaxis associated with use of acetaminophen. Clinical signs included swelling of the face, mouth, and throat, respiratory distress, urticaria, rash, pruritus, and vomiting. There were infrequent reports of life-threatening anaphylaxis requiring emergency medical attention. Instruct patients to discontinue Zamicet® immediately and seek medical care if they experience these symptoms. Do not prescribe Zamicet® for patients with acetaminophen allergy.
Respiratory Depression
At high doses or in sensitive patients, hydrocodone may produce dose-related respiratory depression by acting directly on the brain stem respiratory center. Hydrocodone also affects the center that controls respiratory rhythm, and may produce irregular and periodic breathing.
Infants may have increased sensitivity to the respiratory depressant effects of opioids (see PRECAUTIONS, Pediatric Use). If use of hydrocodone bitartrate and acetaminophen oral solution in such patients is contemplated, it should be administered cautiously, in substantially reduced initial doses, by personnel experienced in administering opioids to infants, and with intensive monitoring.
Head Injury and Increased Intracranial Pressure
The respiratory depressant effects of narcotics and their capacity to elevate cerebrospinal fluid pressure may be markedly exaggerated in the presence of head injury, other intracranial lesions or a preexisting increase in intracranial pressure. Furthermore, narcotics produce adverse reactions which may obscure the clinical course of patients with head injuries.
Acute Abdominal Conditions
The administration of narcotics may obscure the diagnosis or clinical course of patients with acute abdominal conditions.
Misuse, Abuse and Diversion of Opioids
Zamicet® contains hydrocodone, an opioid agonist, and is a Schedule II controlled substance. Opioid agonists have the potential for being abused and are sought by abusers and people with addiction disorders, and are subject to diversion.
Zamicet® can be abused in a manner similar to other opioid agonists, legal or illicit. This should be considered when prescribing or dispensing Zamicet® in situations where the physician or pharmacist is concerned about an increased risk of misuse, abuse or diversion (see DRUG ABUSE AND DEPENDENCE).
Precautions
General
Special Risk Patients
As with any narcotic analgesic agent, Zamicet® should be used with caution in elderly or debilitated patients, and those with severe impairment of hepatic or renal function, hypothyroidism, Addison's disease, prostatic hypertrophy or urethral stricture. The usual precautions should be observed and the possibility of respiratory depression should be kept in mind.
Information for Patients/Caregivers
- Do not take Zamicet® if you are allergic to any of its ingredients.
- If you develop signs of allergy such as a rash or difficulty breathing stop taking Zamicet® and contact your healthcare provider immediately.
- Do not take more than 4000 milligrams of acetaminophen per day. Call your doctor if you took more than the recommended dose.
Hydrocodone, like all narcotics, may impair mental and/or physical abilities required for the performance of potentially hazardous tasks such as driving a car or operating machinery. Such tasks should be avoided while taking this product.
Alcohol and other CNS depressants may produce an additive CNS depression, when taken with this combination product, and should be avoided.
Hydrocodone may be habit-forming. Patients should take the drug only for as long as it is prescribed, in the amounts prescribed, and no more frequently than prescribed.
Physicians should instruct patients and caregivers to read the patient information leaflet, which appears as the last section of the labeling.
Laboratory Tests
In patients with severe hepatic or renal disease, effects of therapy should be monitored with serial liver and/or renal function tests.
Drug Interactions
Patients receiving narcotics, antihistamines, antipsychotics, antianxiety agents, or other CNS depressants (including alcohol) concomitantly with Zamicet® may exhibit an additive CNS depression. When combined therapy is contemplated, the dose of one or both agents should be reduced.
The use of MAO inhibitors or tricyclic antidepressants with hydrocodone preparations may increase the effect of either the antidepressant or hydrocodone.
Drug/Laboratory Test Interactions
Acetaminophen may produce false-positive test results for urinary 5-hydroxyindoleacetic acid.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No adequate studies have been conducted in animals to determine whether hydrocodone has a potential for carcinogenesis, mutagenesis, or impairment of fertility.
Hydrocodone has not demonstrated mutagenic potential using the Ames Salmonella-Microsomal Activation test, the Basc test on Drosophila germ cells, and the Micronucleus test on mouse bone marrow.
No adequate studies have been conducted in animals to determine whether acetaminophen has a potential for carcinogenesis, mutagenesis, or impairment of fertility.
Acetaminophen has not demonstrated mutagenic potential using the Ames Salmonella-Microsomal Activation test, the Basc test on Drosophila germ cells, and the Micronucleus test on mouse bone marrow.
Pregnancy
Nonteratogenic Effects
Babies born to mothers who have been taking opioids regularly prior to delivery will be physically dependent. The withdrawal signs include irritability and excessive crying, tremors, hyperactive reflexes, increased respiratory rate, increased stools, sneezing, yawning, vomiting, and fever. These signs usually appear during the first few days of life. The intensity of the syndrome does not always correlate with the duration of maternal opioid use or dose. There is no consensus on the best method of managing withdrawal.
Labor and Delivery
Narcotic analgesics cross the placental barrier. The closer to delivery and the larger the dose used, the greater the possibility of respiratory depression in the newborn. Narcotic analgesics should be avoided during labor if delivery of a premature infant is anticipated. If the mother has received narcotic analgesics during labor, newborn infants should be observed closely for signs of respiratory depression. Resuscitation may be required (see OVERDOSAGE). The effect of hydrocodone, if any, on the later growth, development, and functional maturation of the child is unknown.
Nursing Mothers
Acetaminophen is excreted in breast milk in small amounts, but the significance of its effects on nursing infants is not known. It is not known whether hydrocodone is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from hydrocodone and acetaminophen, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
Safety and effectiveness in the pediatric population below the age of two years have not been established. Use of hydrocodone bitartrate and acetaminophen in the pediatric population is supported by the evidence from adequate and well controlled studies of hydrocodone and acetaminophen combination products in adults with additional data which support the development of metabolic pathways in children two years of age and over (see DOSAGE AND ADMINISTRATION for pediatric dosage information).
Geriatric Use
Clinical studies of hydrocodone bitartrate and acetaminophen oral solution did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Hydrocodone and the major metabolites of acetaminophen are known to be substantially excreted by the kidney. Thus the risk of toxic reactions may be greater in patients with impaired renal function due to the accumulation of the parent compound and/or metabolites in the plasma. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Hydrocodone may cause confusion and over-sedation in the elderly; elderly patients generally should be started on low doses of hydrocodone bitartrate and acetaminophen oral solution and observed closely.
Adverse Reactions/Side Effects
Potential effects of high dosage are also listed in the OVERDOSAGE section.
Cardio-renal: Bradycardia, cardiac arrest, circulatory collapse, renal toxicity, renal tubular necrosis, hypotension.
Central Nervous System/Psychiatric: Anxiety, dizziness, drowsiness, dysphoria, euphoria, fear, general malaise, impairment of mental and physical performance, lethargy, light-headedness, mental clouding, mood changes, psychological dependence, sedation, somnolence progressing to stupor or coma.
Endocrine: Hypoglycemic coma.
Gastrointestinal System: Abdominal pain, constipation, gastric distress, heartburn, hepatic necrosis, hepatitis, occult blood loss, nausea, peptic ulcer, and vomiting.
Genitourinary System: Spasm of vesical sphincters, ureteral spasm, and urinary retention.
Hematologic: Agranulocytosis, hemolytic anemia, iron deficiency anemia, prolonged bleeding time, thrombocytopenia.
Hypersensitivity: Allergic reactions.
Musculoskeletal: Skeletal muscle flaccidity.
Respiratory Depression: Acute airway obstruction, apnea, dose-related respiratory depression (see OVERDOSAGE), shortness of breath.
Special Senses: Cases of hearing impairment or permanent loss have been reported predominantly in patients with chronic overdose.
Skin: Cold and clammy skin, diaphoresis, pruritus, rash.
Related/similar drugs
Drug Abuse and Dependence
Misuse, Abuse and Diversion of Opioids
Zamicet® contains hydrocodone, an opioid agonist, and is a Schedule II controlled substance. Zamicet®, and other opioids used in analgesia can be abused and are subject to criminal diversion.
Addiction is a primary, chronic, neurobiologic disease, with genetic, psychosocial, and environmental factors influencing its development and manifestations. It is characterized by behaviors that include one or more of the following: impaired control over drug use, compulsive use, continued use despite harm, and craving. Drug addiction is a treatable disease utilizing a multidisciplinary approach, but relapse is common.
"Drug seeking" behavior is very common in addicts and drug abusers. Drug-seeking tactics include emergency calls or visits near the end of office hours, refusal to undergo appropriate examination, testing or referral, repeated "loss" of prescriptions, tampering with prescriptions and reluctance to provide prior medical records or contact information for other treating physician(s). "Doctor shopping" to obtain additional prescriptions is common among drug abusers and people suffering from untreated addiction.
Abuse and addiction are separate and distinct from physical dependence and tolerance. Physical dependence usually assumes clinically significant dimensions only after several weeks of continued opioid use, although a mild degree of physical dependence may develop after a few days of opioid therapy. Tolerance, in which increasingly large doses are required in order to produce the same degree of analgesia, is manifested initially by a shortened duration of analgesic effect, and subsequently by decreases in the intensity of analgesia. The rate of development of tolerance varies among patients. Physicians should be aware that abuse of opioids can occur in the absence of true addiction and is characterized by misuse for non-medical purposes, often in combination with other psychoactive substances. Hydrocodone bitartrate and acetaminophen, like other opioids, may be diverted for non-medical use. Record-keeping of prescribing information, including quantity, frequency, and renewal requests is strongly advised.
Proper assessment of the patient, proper prescribing practices, periodic re-evaluation of therapy, and proper dispensing and storage are appropriate measures that help to limit abuse of opioid drugs.
Overdosage
Following an acute overdosage, toxicity may result from hydrocodone or acetaminophen.
Signs and Symptoms
Hydrocodone
Serious overdose with hydrocodone is characterized by respiratory depression (a decrease in respiratory rate and/or tidal volume, Cheyne-Stokes respiration, cyanosis), extreme somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, and sometimes bradycardia and hypotension. In severe overdosage, apnea, circulatory collapse, cardiac arrest and death may occur.
Acetaminophen
In acetaminophen overdosage: dose-dependent, potentially fatal hepatic necrosis is the most serious adverse effect. Renal tubular necrosis, hypoglycemic coma and coagulation defects may also occur.
Early symptoms following a potentially hepatotoxic overdose may include: nausea, vomiting, diaphoresis and general malaise. Clinical and laboratory evidence of hepatic toxicity may not be apparent until 48 to 72 hours post-ingestion.
Treatment
A single or multiple drug overdose with hydrocodone and acetaminophen is a potentially lethal polydrug overdose, and consultation with a regional poison control center is recommended.
Immediate treatment includes support of cardiorespiratory function and measures to reduce drug absorption. Oxygen, intravenous fluids, vasopressors, and other supportive measures should be employed as indicated. Assisted or controlled ventilation should also be considered.
For hydrocodone overdose, primary attention should be given to the reestablishment of adequate respiratory exchange through provision of a patent airway and the institution of assisted or controlled ventilation. The narcotic antagonist naloxone hydrochloride is a specific antidote against respiratory depression which may result from overdosage or unusual sensitivity to narcotics, including hydrocodone. Since the duration of action of hydrocodone may exceed that of the antagonist, the patient should be kept under continued surveillance, and repeated doses of the antagonist should be administered as needed to maintain adequate respiration. A narcotic antagonist should not be administered in the absence of clinically significant respiratory or cardiovascular depression.
Gastric decontamination with activated charcoal should be administered just prior to N-acetylcysteine (NAC) to decrease systemic absorption if acetaminophen ingestion is known or suspected to have occurred within a few hours of presentation. Serum acetaminophen levels should be obtained immediately if the patient presents 4 hours or more after ingestion to assess potential risk of hepatoxicity; acetaminophen levels drawn less than 4 hours post-ingestion may be misleading. To obtain the best possible outcome, NAC should be administered as soon as possible where impending or evolving liver injury is suspected. Intravenous NAC may be administered when circumstances preclude oral administration.
Vigorous supportive therapy is required in severe intoxication. Procedures to limit the continuing absorption of the drug must be readily performed since the hepatic injury is dose dependent and occurs early in the course of intoxication.
Zamicet Dosage and Administration
Dosage should be adjusted according to the severity of the pain and the response of the patient. However, it should be kept in mind that tolerance to hydrocodone can develop with continued use and that the incidence of untoward effects is dose related.
The usual adult dosage is one tablespoonful (15 mL) every four to six hours as needed for pain. The total daily dosage should not exceed 6 tablespoonfuls.
The usual dosages for children are given by the table below, and are to be given every 4 to 6 hours as needed for pain. These dosages correspond to an average individual dose of 0.20 mL/kg of Zamicet® (providing 0.135 mg/kg of hydrocodone bitartrate and 4.38 mg/kg of acetaminophen). Dosing should be based on weight whenever possible.
| BODY WEIGHT | APPROXIMATE AGE | DOSE every 4 to 6 hours | MAXIMUM TOTAL DAILY DOSE (6 doses per day) |
|---|---|---|---|
| 12 to 15 kg (27 to 34 lbs) | 2 to 3 years | 2.8 mL (approx. ½ teaspoonful) | 16.8 mL (approx. 3¼ teaspoonfuls) |
| 16 to 22 kg (35 to 50 lbs) | 4 to 6 years | 3.75 mL (approx. ¾ teaspoonful) | 22.5 mL (approx. 4½ teaspoonfuls) |
| 23 to 31 kg (51 to 69 lbs) | 7 to 9 years | 5.6 mL (approx. 1 teaspoonful) | 33.6 mL (approx. 6½ teaspoonfuls) |
| 32 to 45 kg (70 to 100 lbs) | 10 to 13 years | 7.5 mL (approx. 1½ teaspoonfuls) | 45 mL (approx. 9 teaspoonfuls) |
| 46 kg and up (101 lbs and up) | 14 years to adult | 11.25 mL (approx. 2¼ teaspoonfuls) | 67.5 mL (approx. 13½ teaspoonfuls) |
| — | adult | 15 mL (1 Tablespoonful) | 90 mL (6 Tablespoonfuls) |
The total daily dosage for children should not exceed 6 doses per day. It is of utmost importance that the dose of Zamicet® be administered accurately. A household teaspoon or tablespoon is not an adequate measuring device, especially when one-half or three-fourths of a teaspoonful is to be measured. Given the inexactitude of the household spoon measure and the possibility of using a tablespoon instead of a teaspoon, which could lead to overdosage, it is strongly recommended that caregivers obtain and use a calibrated measuring device. Health care providers should recommend a dropper that can measure and deliver the prescribed dose accurately, and instruct caregivers to use extreme caution in measuring the dosage.
How is Zamicet supplied
Zamicet® is a yellow-colored, fruit flavored liquid containing 10 mg hydrocodone bitartrate and 325 mg acetaminophen per 15 mL, with 6.7% alcohol. It is supplied in the following oral dosage forms:
NDC 0121-1542-07: 7.5 mL unit dose cup
NDC 0121-1542-40: Case contains 40 unit dose cups of 7.5 mL (0121-1542-07) packaged in 4 trays of 10 unit dose cups each.
NDC 0121-2313-15: 15 mL unit dose cup
NDC 0121-2313-40: Case contains 40 unit dose cups of 15 mL (0121-2313-15) packaged in 4 trays of 10 unit dose cups each.
Patient Information Leaflet
Zamicet® CII
(Hydrocodone Bitartrate and Acteaminophen Oral Solution)
10 mg/325 mg per 15 mL
Rx ONLY
Summary
| Zamicet® (hydrocodone bitartrate and acetaminophen oral solution) is used to relieve moderate to moderately severe pain. You should not take this medicine if you are allergic to hydrocodone or acetaminophen. The most common side effects of this medicine are abdominal pain, dizziness, drowsiness, light-headedness, nausea, shortness of breath, unusual tiredness, and vomiting. Take this medicine as directed by your doctor. Do not take more of it, do not take it more often, and do not take it for a longer time than your doctor ordered. |
Uses
Zamicet® is an analgesic used to relieve moderate to moderately severe pain. Zamicet® is a combination product containing hydrocodone (hye-droe-KO-done) bitartrate and acetaminophen (a-seat-a-MIN-oh-fen). Hydrocodone is a narcotic pain reliever and a cough suppressant. Acetaminophen is a non-narcotic pain reliever and fever reducer. A narcotic analgesic and acetaminophen used together may provide better pain relief than either product used alone. If you have any questions, please call your doctor or pharmacist.
General Cautions
- Do not take this drug if you have allergies or unusual reactions to narcotic pain relievers or acetaminophen because it is likely that you may also be allergic to Zamicet®.
- This product may inhibit your mental and physical abilities required for the performance of potentially hazardous tasks such as driving a car or operating machinery. Such tasks should be avoided while you are taking this product.
- This medicine may not be right for you. Check with your doctor or pharmacist, if you:
- are pregnant.
- are nursing.
- are taking other medications: narcotic pain relievers; allergy medicines; antidepressant medicines; acetaminophen-containing medicines or other medicines that cause central nervous system depression, including alcohol.
- have other medical problems: a history of drug or alcohol abuse; recent head injury; emphysema, asthma, or other chronic lung disease; liver disease; kidney disease; underactive thyroid, Addison's disease, enlarged prostate or difficulty urinating.
Proper Use
Take this medicine as directed by your doctor. Do not share it with anyone else. This medicine can cause drug dependence and has the potential for abuse. Do not take more of it, do not take it more often, and do not take it for a longer time than your doctor ordered. If you think that this medicine is not working properly after taking it for some time, do not increase the dose. Check with your doctor or pharmacist.
Dosing
The dose of this medication will be different for different patients. Follow the directions provided by your doctor. The following information includes only the average dose of this medication. If your dose is different, do not change doses unless your doctor tells you to do so.
| BODY WEIGHT | APPROXIMATE AGE | DOSE every 4 to 6 hours | MAXIMUM TOTAL DAILY DOSE (6 doses per day) |
|---|---|---|---|
| 12 to 15 kg (27 to 34 lbs) | 2 to 3 years | 2.8 mL (approx. ½ teaspoonful) | 16.8 mL (approx. 3¼ teaspoonfuls) |
| 16 to 22 kg (35 to 50 lbs) | 4 to 6 years | 3.75 mL (approx. ¾ teaspoonful) | 22.5 mL (approx. 4½ teaspoonfuls) |
| 23 to 31 kg (51 to 69 lbs) | 7 to 9 years | 5.6 mL (approx. 1 teaspoonful) | 33.6 mL (approx. 6½ teaspoonfuls) |
| 32 to 45 kg (70 to 100 lbs) | 10 to 13 years | 7.5 mL (approx. 1½ teaspoonfuls) | 45 mL (approx. 9 teaspoonfuls) |
| 46 kg and up (101 lbs and up) | 14 years to adult | 11.25 mL (approx. 2¼ teaspoonfuls) | 67.5 mL (approx. 13½ teaspoonfuls) |
| — | adult | 15 mL (1 Tablespoonful) | 90 mL (6 Tablespoonfuls) |
It is very important that Zamicet® be dosed accurately. A household teaspoon or tablespoon is not an accurate measuring device, especially when one-half or three-fourths of a teaspoonful is to be measured.
Since a household teaspoon is not accurate and can be mixed-up with a tablespoon (which can cause overdosage), it is strongly recommended that you obtain and use a proper measuring device. Ask your doctor or pharmacist for help to find a dropper that can measure the needed dose properly and ask for help if you do not understand how to use the dropper.
Missed Dose
- To avoid a possible overdose, it is important that you do not take more than a single dosage at one time, or that you don't take doses at intervals less than 4 hours apart.
- If you miss taking a dose of Zamicet®, take it as soon as you remember. However, make sure to wait at least 4 hours before taking your next dose.
- If you missed taking a dose, and it is almost time for your next dose, skip the missed dose and take your medicine as scheduled.
- Do not double the prescribed dose.
Possible Side Effects
Side effects you may experience include abdominal pain, constipation, difficulty urinating, dizziness, drowsiness, fear, fuzzy thinking, general feeling of discomfort or illness, light-headedness, mood changes, nausea, nervousness, rash, shortness of breath, slower reactions, unusual tiredness, and vomiting.
Call your doctor if these effects continue or are bothersome.
Side effects not listed above may sometimes occur. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Storage
- Keep out of reach of children.
- Store at room temperature (protect from heat, do not refrigerate).
- Keep in original labeled bottle.
- Discard medicines that are old or no longer needed.
- Even a single overdose of this medicine may be a life-threatening situation. If you suspect that you or someone else may have taken more than the prescribed dose of this medicine, contact your local poison control center or emergency room immediately. This medicine was prescribed for your particular condition. Do not use it for another condition or give the drug to others.
- This leaflet provides a summary of information about Zamicet®. If you have any questions or concerns, or want more information about Zamicet®, contact your doctor or pharmacist. Your pharmacist also has a longer leaflet about Zamicet® that is written for health professionals that you can ask to read.
PRINCIPAL DISPLAY PANEL - 7.5 mL Cup Label
Delivers 7.5 mL
NDC 0121-1542-07
Zamicet®
(Hydrocodone Bitartrate and
Acetaminophen Oral Solution)
CII
5 mg/163 mg per 7.5 mL
Alcohol 6.7%
FOR INSTITUTIONAL USE ONLY
See Package Insert for Dosage
Rx ONLY
PHARMACEUTICAL ASSOCIATES, INC
GREENVILLE, SC 29605
A4771R0704

PRINCIPAL DISPLAY PANEL - 15 mL Cup Label
Delivers 15 mL
NDC 0121-2313-15
Zamicet®
(Hydrocodone Bitartrate and
Acetaminophen Oral Solution)
CII
10 mg/325 mg per 15 mL
Alcohol 6.7%
FOR INSTITUTIONAL USE ONLY
See Package Insert for Dosage
Rx ONLY
PHARMACEUTICAL ASSOCIATES, INC
GREENVILLE, SC 29605
A4771R1504

| ZAMICET
hydrocodone bitartrate and acetaminophen solution |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| ZAMICET
hydrocodone bitartrate and acetaminophen solution |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Pharmaceutical Associates, Inc. (044940096) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pharmaceutical Associates, Inc. | 097630693 | MANUFACTURE(0121-1542, 0121-2313) | |
More about Zamicet (acetaminophen / hydrocodone)
- Check interactions
- Compare alternatives
- Side effects
- Dosage information
- During pregnancy
- Drug class: narcotic analgesic combinations
Patient resources
Professional resources
Other brands
Norco, Vicodin, Lortab, Hycet, ... +7 more
