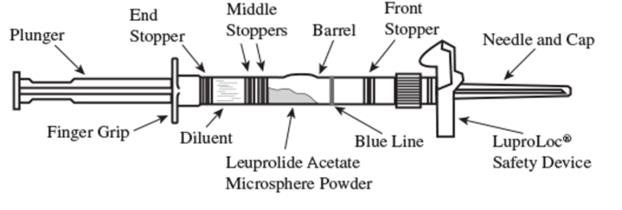
Lupron Depot Dosage
Generic name: LEUPROLIDE ACETATE 45mg in 1.5mL;
Dosage form: injection
Drug classes: Gonadotropin releasing hormones, Hormones / antineoplastics
Medically reviewed by Drugs.com. Last updated on Mar 12, 2024.
LUPRON DEPOT must be administered under the supervision of a physician.
In patients treated with GnRH analogues for prostate cancer, treatment is usually continued upon development of non-metastatic and metastatic castration-resistant prostate cancer.
| Dosage | 7.5 mg for 1-Month Administration | 22.5 mg for 3-Month Administration | 30 mg for 4-Month Administration | 45 mg for 6-Month Administration |
| Recommended dose | 1 injection every 4 weeks | 1 injection every 12 weeks | 1 injection every 16 weeks | 1 injection every 24 weeks |
2.1 LUPRON DEPOT 7.5 mg for 1-Month Administration
The recommended dose of LUPRON DEPOT 7.5 mg for 1-month administration is one injection every 4 weeks. Do not use concurrently a fractional dose, or a combination of doses of this or any depot formulation due to different release characteristics.
Incorporated in a depot formulation, the lyophilized microspheres must be reconstituted and should be administered every 4 weeks as a single intramuscular injection.
For optimal performance of the prefilled dual chamber syringe (PDS), read and follow the instructions in Section 2.5.
2.2 LUPRON DEPOT 22.5 mg for 3-Month Administration
The recommended dose of LUPRON DEPOT 22.5 mg for 3-month administration is one injection every 12 weeks. Do not use concurrently a fractional dose, or a combination of doses of this or any depot formulation due to different release characteristics.
Incorporated in a depot formulation, the lyophilized microspheres must be reconstituted and should be administered every 12 weeks as a single intramuscular injection.
For optimal performance of the prefilled dual chamber syringe (PDS), read and follow the instructions in Section 2.5.
2.3 LUPRON DEPOT 30 mg for 4-Month Administration
The recommended dose of LUPRON DEPOT 30 mg for 4-month administration is one injection every 16 weeks. Do not use concurrently a fractional dose, or a combination of doses of this or any depot formulation due to different release characteristics.
Incorporated in a depot formulation, the lyophilized microspheres must be reconstituted and should be administered every 16 weeks as a single intramuscular injection.
For optimal performance of the prefilled dual chamber syringe (PDS), read and follow the instructions in Section 2.5.
2.4 LUPRON DEPOT 45 mg for 6-Month Administration
The recommended dose of LUPRON DEPOT 45 mg for 6-month administration is one injection every 24 weeks. Do not use concurrently a fractional dose, or a combination of doses of this or any depot formulation due to different release characteristics.
Incorporated in a depot formulation, the lyophilized microspheres must be reconstituted and should be administered every 24 weeks as a single intramuscular injection.
For optimal performance of the prefilled dual chamber syringe (PDS), read and follow the instructions in Section 2.5.
2.5 Reconstitution and Administration for Injection of LUPRON DEPOT
- Reconstitute and administer the lyophilized microspheres as a single intramuscular injection.
- Inject the suspension immediately or discard if not used within two hours, because LUPRON DEPOT does not contain a preservative.
1. Visually inspect the LUPRON DEPOT powder. DO NOT USE the syringe if clumping or caking is evident. A thin layer of powder on the wall of the syringe is considered normal prior to mixing with the diluent. The diluent should appear clear and colorless.
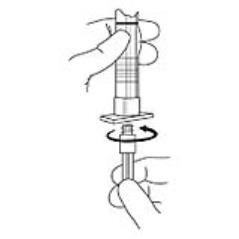
2. To prepare for injection, screw the white plunger into the end stopper until the stopper begins to turn (see Figure 1 and Figure 2).
Figure 1
Figure 2
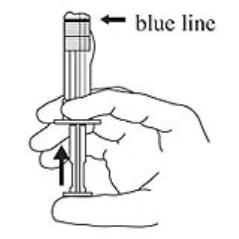
3. Hold the syringe UPRIGHT. Release the diluent by SLOWLY PUSHING (6 to 8 seconds) the plunger until the first middle stopper is at the blue line in the middle of the barrel (see Figure 3).
Figure 3
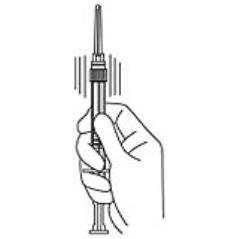
4. Keep the syringe UPRIGHT. Mix the microspheres (powder) thoroughly by gently shaking the syringe until the powder forms a uniform suspension. The suspension will appear milky. If the powder adheres to the stopper or caking/clumping is present, tap the syringe with your finger to disperse. DO NOT USE if any of the powder has not gone into suspension (see Figure 4).
Figure 4
5. Keep the syringe UPRIGHT. With the opposite hand pull the needle cap upward without twisting.
6. Keep the syringe UPRIGHT. Advance the plunger to expel the air from the syringe. Now the syringe is ready for injection.
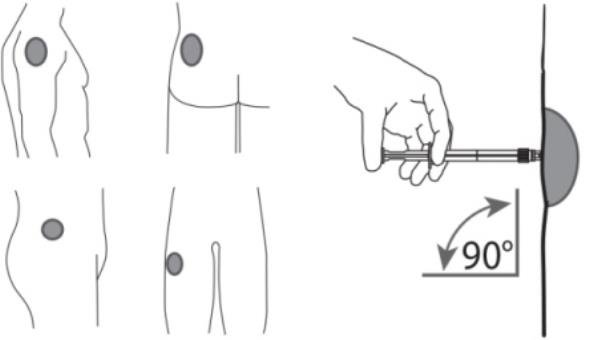
7. After cleaning the injection site with an alcohol swab, administer the intramuscular injection by inserting the needle at a 90 degree angle into the gluteal area, anterior thigh, or deltoid; injection sites should be alternated (see Figure 5).
Figure 5
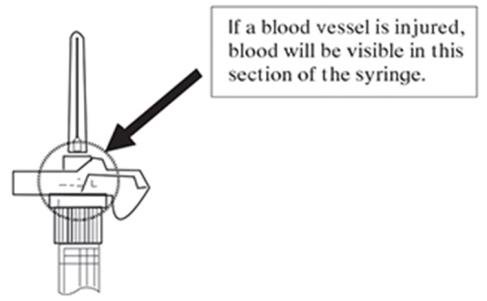
NOTE: If a blood vessel is accidentally penetrated, aspirated blood will be visible just below the luer lock (see Figure 6) and can be seen through the transparent LuproLoc® safety device. If blood is present, remove the needle immediately. Do not inject the medication.
Figure 6
8. Inject the entire contents of the syringe intramuscularly.
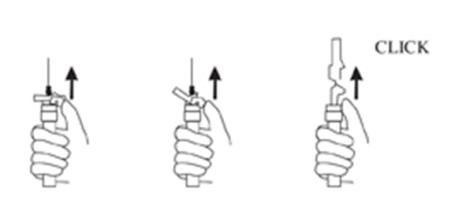
9. Withdraw the needle. Once the syringe has been withdrawn, immediately activate the LuproLoc® safety device by pushing the arrow on the lock upward towards the needle tip with the thumb or finger, as illustrated, until the needle cover of the safety device over the needle is fully extended and a CLICK is heard or felt (see Figure 7).
Figure 7
10. Dispose of the syringe according to local regulations/procedures.
Frequently asked questions
- How long should you take Lupron for prostate cancer?
- Are Lupron Depot and Eligard the same drug?
- Can you get pregnant on Lupron Depot?
- Is Lupron Depot a chemotherapy treatment?
- Does Lupron Depot need to be refrigerated?
- Is Firmagon (degarelix) the same as Lupron Depot?
- How do you inject Lupron Depot for prostate cancer?
- Will I get my period while on Lupron?
- What does Lupron do for IVF?
More about Lupron Depot (leuprolide)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (242)
- Drug images
- Side effects
- Patient tips
- During pregnancy
- Support group
- Drug class: gonadotropin releasing hormones
- En español
Patient resources
Other brands
Eligard, Camcevi, Fensolvi, Viadur
Professional resources
Other brands
Eligard, Camcevi, Lupron Depot-Gyn, Fensolvi, Lutrate Depot
Other formulations
Related treatment guides
See also:
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.