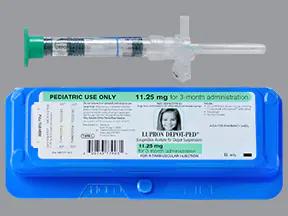
Lupron Depot-PED: Package Insert / Prescribing Info
Package insert / product label
Generic name: leuprolide acetate
Dosage form: injection
Drug classes: Gonadotropin releasing hormones, Hormones / antineoplastics
J Codes (medical billing codes): J1950 (3.75 mg, intramuscular); J9217 (7.5 mg, injection)
Medically reviewed by Drugs.com. Last updated on Jun 4, 2025.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Drug Interactions
- Use In Specific Populations
- Overdosage
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Patient Counseling Information
- Medication Guide
Highlights of Prescribing Information
LUPRON DEPOT-PED (leuprolide acetate for depot suspension), for intramuscular use
Initial U.S. Approval: 1985
Indications and Usage for Lupron Depot-PED
LUPRON DEPOT-PED is a gonadotropin releasing hormone (GnRH) agonist indicated for the treatment of pediatric patients with central precocious puberty. (1)
Lupron Depot-PED Dosage and Administration
- Must be administered by a healthcare professional. (2.1)
- Select appropriate LUPRON DEPOT-PED syringe for the intended dosing frequency and administer intramuscularly. (2.1)
- For 1-month administration: Starting dose is 7.5, 11.25, or 15 mg based on the patient’s weight. (2.2)
- For 3-month administration: Doses are either 11.25 or 30 mg. (2.3)
- For 6-month administration: Dose is 45 mg. (2.4)
- Monitor hormonal and clinical parameters during treatment to ensure adequate suppression. (2.2, 2.3, 2.4)
- Rotate injection site periodically. (2.5)
- See Full Prescribing Information for administration and reconstitution instructions. (2.5, 2.6)
Dosage Forms and Strengths
For depot suspension: leuprolide acetate as a lyophilized powder supplied in single-dose, prefilled dual-chamber syringe with diluent (3):
- For 1-month administration: 7.5 mg, 11.25 mg, or 15 mg
- For 3-month administration: 11.25 mg or 30 mg
- For 6-month administration: 45 mg
Contraindications
Warnings and Precautions
-
Initial Rise of Gonadotropins and Sex Steroid Levels: During the early phase of therapy, gonadotropins and sex steroids may rise above baseline because of the initial stimulatory effect of the drug. Therefore, an increase in clinical signs and symptoms of puberty, including vaginal bleeding, may be observed during the first weeks of therapy or after subsequent doses. (5.1)
-
Psychiatric events: Have been reported in patients taking GnRH agonists. Events include emotional lability, such as crying, irritability, impatience, anger, and aggression. Monitor for development or worsening of psychiatric symptoms. (5.2)
-
Convulsions: Have been observed in patients with or without a history of seizures, epilepsy, cerebrovascular disorders, central nervous system anomalies or tumors, and in patients on concomitant medications that have been associated with convulsions. (5.3)
- Pseudotumor Cerebri (Idiopathic Intracranial Hypertension): Have been reported in pediatric patients receiving GnRH agonists, including LUPRON DEPOT-PED. Monitor patients for headache, papilledema, and blurred vision. (5.4)
Adverse Reactions/Side Effects
- Adverse events related to suppression of endogenous sex steroid secretion and injection site reactions including abscess may occur with LUPRON DEPOT-PED 7.5 mg, 11.25 mg, or 15 mg for 1-month administration. (6.1, 6.2)
- In the clinical studies for LUPRON DEPOT-PED 7.5 mg, 11.25 mg, or 15mg for 1-month administration the most common (≥2%) adverse reactions were: emotional lability, headache, general pain, acne/seborrhea, rash including erythema multiforme and vaginitis/vaginal bleeding/vaginal discharge. (6.1)
- In the clinical studies for LUPRON DEPOT-PED 11.25 mg or 30 mg for 3-month administration the most common (>2%) adverse reactions were: injection site pain, weight increased, headache, mood altered, and injection site swelling. (6.1)
- In the clinical study for LUPRON DEPOT-PED 45 mg for 6-month administration the most common (≥4%) adverse reactions were: injection site reactions, headache, psychiatric events, abdominal pain, diarrhea, hemorrhage, nausea and vomiting, pyrexia, pruritus, pain in extremity, rash, back pain, ligament sprain, weight increased, fracture, breast tenderness, insomnia, chest pain, and hyperhidrosis. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact AbbVie Inc. at 1-800-633-9110 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 5/2025
Full Prescribing Information
1. Indications and Usage for Lupron Depot-PED
LUPRON DEPOT-PED is indicated for the treatment of pediatric patients with central precocious puberty (CPP).
2. Lupron Depot-PED Dosage and Administration
2.1 Important Dosing Information
- LUPRON DEPOT-PED must be administered by a healthcare professional.
- Individualize the dose of LUPRON DEPOT-PED for each patient.
- Select the appropriate LUPRON-DEPOT PED syringe for the intended dosing frequency and administer intramuscularly.
- Each LUPRON DEPOT-PED strength and formulation has different release characteristics. Do not use partial syringes or a combination of syringes to achieve a particular dose.
- In the case of inadequate suppression of pituitary gonadotropins and peripheral sex steroids with a maximal dosage, consider other available gonadotropin releasing hormone (GnRH) agonists indicated for the treatment of central precocious puberty.
- Discontinue LUPRON DEPOT-PED at the appropriate age of onset of puberty.
2.2 Dosage and Recommended Monitoring for 1-Month Administration
- Administer LUPRON DEPOT-PED 7.5 mg, 11.25 mg, or 15 mg for 1-month administration as a single-dose intramuscular injection once every month.
- The starting dose is based on the patient's weight (see Table 1).
| Table 1. Dosage Recommendations Based on Body Weight for LUPRON DEPOT-PED for 1-Month Administration | |
| Body Weight | Once Monthly Recommended Dosage |
| Less than or equal to 25 kg | 7.5 mg |
| Greater than 25 kg up to 37.5 kg | 11.25 mg |
| Greater than 37.5 kg | 15 mg |
- The dosage may need to be adjusted with changes in body weight.
- If adequate hormonal and clinical suppression is not achieved with the starting dose, increase the dosage to the next available higher dose (e.g., 11.25 mg or 15 mg at the next monthly injection).
- Monitor response with a GnRH stimulation test, basal luteinizing hormone (LH) or serum concentration of sex steroid levels beginning 1 to 2 months following initiation of therapy, with changing doses, or further as judged clinically appropriate in order to confirm maintenance of efficacy.
- Assess height (for calculation of growth rate) and bone age every 6 to 12 months.
2.3 Dosage and Recommended Monitoring for 3-Month Administration
- Use LUPRON DEPOT-PED 11.25 mg or 30 mg for 3-month administration once every three months (12 weeks) as a single-dose intramuscular injection.
- Monitor response with a GnRH stimulation test, basal LH or serum concentration of sex steroid levels at months 2 to 3, month 6 and further as judged clinically appropriate, to confirm maintenance of efficacy.
- Assess height (for calculation of growth rate) and bone age every 6 to 12 months.
2.4 Dosage and Recommended Monitoring for 6-Month Administration
- Use LUPRON DEPOT-PED 45 mg for 6-month administration once every six months (24 weeks) as a single-dose intramuscular injection.
- Monitor response with a GnRH stimulation test, basal LH or serum concentration of sex steroid levels at months 5 to 6 and further as judged clinically appropriate, to confirm maintenance of efficacy.
- Assess height (for calculation of growth rate) and bone age every 6 to 12 months.
2.5 Important Administration Instructions
- Administer LUPRON DEPOT-PED as a single-dose intramuscular injection into the gluteal area, anterior thigh, or shoulder.
- Rotate injection sites within the same region from one injection to the next.
- Inject immediately after reconstitution. Discard if not used within 2 hours.
2.6 Reconstitution Instructions
1. Visually inspect the LUPRON DEPOT-PED powder and diluent. Do not use the syringe if clumping or caking is evident. A thin layer of powder on the wall of the syringe is considered normal prior to mixing with the diluent. The diluent should appear clear and free from particulate matter. Do not use the diluent if it is not clear or there is particulate matter.
2. To prepare for injection, screw the white plunger into the end stopper until the stopper begins to turn. (see Figure 1 and Figure 2)
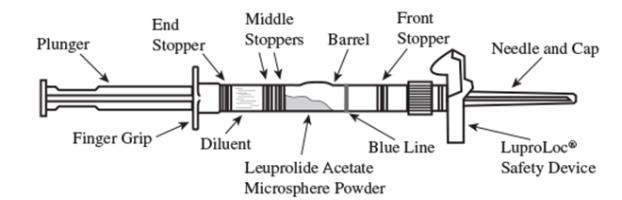
Figure 1
LuproLoc Safety Device should be activated after product injection, refer to Step 9 (Figure 7).
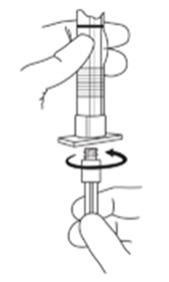
Figure 2
3. Hold the syringe upright. Release the diluent by slowly pushing the plunger for 6 to 8 seconds until the first stopper is at the blue line in the middle of the barrel. (Figure 3)
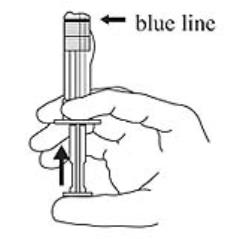
Figure 3
4. Keep the syringe upright. Mix the powder thoroughly by gently shaking the syringe until the powder forms a uniform suspension. The suspension will appear milky. If the powder adheres to the stopper or caking/clumping is present, tap the syringe with your finger to disperse. Do not use if any of the powder has not gone into suspension. (Figure 4)
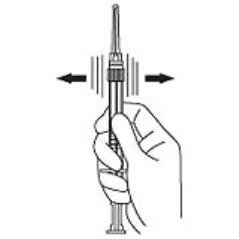
Figure 4
5. Hold the syringe upright. With the opposite hand pull the needle cap upward without twisting.
6. Keep the syringe upright. Advance the plunger to expel the air from the syringe.
Now the syringe is ready for injection.
7. After cleaning the injection site with an alcohol swab, administer the intramuscular injection by inserting the needle at a 90 degree angle into the deltoid, gluteal area, or anterior thigh. (Figure 5)
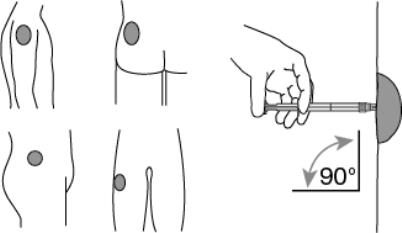
Figure 5
NOTE: Aspirated blood would be visible just below the luer lock connection if a blood vessel is accidentally penetrated. If present, blood can be seen through the transparent LuproLoc® safety device. If blood is present remove the needle immediately. Do not inject the medication. (Figure 6)
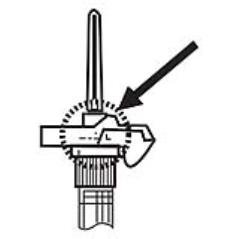
Figure 6
8. Inject the entire contents of the syringe intramuscularly immediately after reconstitution. The suspension settles very quickly following reconstitution.
9. Withdraw the needle. Once the syringe has been withdrawn, activate immediately the LuproLoc® safety device by pushing the arrow on the lock upward towards the needle tip with the thumb or finger, as illustrated, until the needle cover of the safety device is fully extended over the needle and a click is heard or felt. (Figure 7)
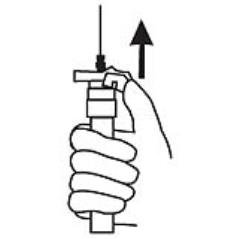
Figure 7
3. Dosage Forms and Strengths
For depot suspension: a white lyophilized powder supplied in a single-dose, prefilled dual-chamber syringe with a colorless diluent is available as:
- For 1-month administration: 7.5 mg, 11.25 mg, or 15 mg of leuprolide acetate
- For 3-month administration: 11.25 mg or 30 mg of leuprolide acetate
- For 6-month administration: 45 mg of leuprolide acetate
5. Warnings and Precautions
5.1 Initial Rise of Gonadotropins and Sex Steroid Levels
During the early phase of therapy or after subsequent doses, gonadotropins and sex steroids may rise above baseline because of a transient stimulatory effect of the drug [see Clinical Pharmacology (12.2)]. Therefore, an increase in clinical signs and symptoms of puberty, including vaginal bleeding, may be observed during the first weeks of therapy or after subsequent doses [see Adverse Reactions (6)].
5.2 Psychiatric Events
Psychiatric events have been reported in patients taking GnRH agonists, including LUPRON DEPOT-PED. Postmarking reports with this class of drugs include symptoms of emotional lability, such as crying, irritability, impatience, anger and aggression. Monitor for development or worsening of psychiatric symptoms during treatment with LUPRON DEPOT-PED [see Adverse Reactions (6.2)].
5.3 Convulsions
Postmarketing reports of convulsions have been observed in patients receiving GnRH agonists, including LUPRON DEPOT-PED. These included patients with a history of seizures, epilepsy, cerebrovascular disorders, central nervous system anomalies or tumors, and patients on concomitant medications that have been associated with convulsions such as bupropion and SSRIs. Convulsions have also been reported in patients in the absence of any of the conditions mentioned above [see Adverse Reactions (6.2)].
5.4 Pseudotumor Cerebri (Idiopathic Intracranial Hypertension)
Pseudotumor cerebri (idiopathic intracranial hypertension) have been reported in pediatric patients receiving GnRH agonists, including LUPRON DEPOT-PED. Monitor patients for signs and symptoms of pseudotumor cerebri, including headache, papilledema, blurred vision, diplopia, loss of vision, pain behind the eye or pain with eye movement, tinnitus, dizziness, and nausea.
6. Adverse Reactions/Side Effects
The following serious adverse reactions are described here and elsewhere in the label:
- Initial rise in gonadotropin and sex steroid levels [see Warnings and Precautions (5.1)].
- Psychiatric Events [see Warnings and Precautions (5.2)].
- Convulsions [see Warnings and Precautions (5.3)].
- Pseudotumor Cerebri (Idiopathic Intracranial Hypertension) [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
LUPRON DEPOT-PED for 1-month administration
LUPRON DEPOT-PED 1-month administration was evaluated in a pivotal, open label, multicenter study in which 55 (49 female and 6 male) pediatric patients with central precocious puberty were enrolled. The age ranged from 1 to 8 years of age at the beginning of treatment; the mean age for females was 6.8 years (range: 1 to 9 years) and the mean age for males was 7.5 years (range: 4 to 9 years); 61.8% were Caucasian; 20% Black; 1.8% Oriental; and 16.4% Hispanic.
Adverse reactions that occurred in ≥2% of patients are shown in Table 2.
| Table 2. Adverse Reactions Occurring in ≥2% in Pediatric Patients with CPP Receiving
LUPRON DEPOT-PED 1-month |
|
| % of Patients
(N = 421) |
|
| Injection Site Reactions Including Abscess* | 9 |
| Emotional Lability | 5 |
| Headache | 3 |
| General Pain | 3 |
| Acne/Seborrhea | 3 |
| Rash Including Erythema Multiforme | 3 |
| Vaginitis/Vaginal Bleeding/Vaginal Discharge | 3 |
| Vasodilation | 2 |
| * Most events were mild or moderate in severity. | |
Less Common Adverse Reactions
The following adverse reactions were reported in less than 2% of the patients and are listed below by body system.
Body as a Whole – aggravation of preexisting tumor and decreased vision, allergic reaction, body odor, fever, flu syndrome, hypertrophy, infection;
Cardiovascular System – bradycardia, hypertension, peripheral vascular disorder, syncope; Digestive System – constipation, dyspepsia, dysphagia, gingivitis, increased appetite, nausea/vomiting;
Endocrine System – accelerated sexual maturity, feminization, goiter;
Hemic and Lymphatic System – purpura;
Metabolic and Nutritional Disorders – growth retarded, peripheral edema, weight gain; Musculoskeletal System – arthralgia, joint disorder, myalgia, myopathy;
Nervous System – hyperkinesia, somnolence;
Psychiatric System – depression, nervousness;
Respiratory System – asthma, epistaxis, pharyngitis, rhinitis, sinusitis;
Integumentary System (Skin and Appendages) – alopecia, hair disorder, hirsutism, leukoderma, nail disorder, skin hypertrophy;
Urogenital System – cervix disorder/neoplasm, dysmenorrhea, gynecomastia/breast disorders, menstrual disorder, urinary incontinence.
Laboratory: The following laboratory events were reported as adverse reactions: antinuclear antibody present and increased sedimentation rate.
LUPRON DEPOT-PED for 3-month administration
LUPRON DEPOT-PED for 3-month administration was evaluated in a pivotal, open-label, multicenter, clinical study with 84 randomized pediatric patients with central precocious puberty; 76 (90.5%) were females and 8 (9.5%) were males. The age ranged from 1 to 11 years age at the beginning of treatment; 80/84 (95.2%) were 5 years or older, and female patients were younger than male; the mean age for 11.25 mg and 30 mg groups for females was 7.6 and 7.7 years, and for males 9.3 and 9.4 years, respectively; 58.3% were Caucasian; 22.6% were Black; 7.1% were Asian; 1.2% were Native Hawaiian or Other Pacific Islander; and 10.7% were Multi-race.
Adverse reactions that occurred in ≥2% of patients are shown in Table 3.
| Table 3. Adverse Reactions Occurring in ≥2% in Pediatric Patients with CPP
Receiving LUPRON DEPOT-PED for 3-month administration. |
|||
| %
11.25 mg every 3 Months N=42 | %
30 mg every 3 Months N=42 | %
Overall N = 84 |
|
| Injection site pain | 19 | 21 | 20 |
| Weight increased | 7 | 7 | 7 |
| Headache | 2 | 7 | 5 |
| Mood altered | 5 | 5 | 5 |
| Injection site swelling | 2 | 2 | 2 |
Less Common Adverse Reactions
The following adverse reactions were reported in one patient and are listed below by system organ class:
Gastrointestinal Disorders – abdominal pain, nausea;
General Disorders and Administration Site Conditions – asthenia, gait disturbance, injection site abscess sterile, injection site hematoma, injection site induration, injection site warmth, irritability;
Metabolic and Nutritional Disorders – decreased appetite, obesity;
Musculoskeletal and Connective Tissue Disorders - musculoskeletal pain, pain in extremity; Nervous System Disorders – dizziness;
Psychiatric Disorders – crying, tearfulness;
Respiratory, Thoracic and Mediastinal Disorders – cough;
Skin and Subcutaneous Tissue Disorders – hyperhidrosis;
Vascular Disorders – pallor.
LUPRON DEPOT-PED for 6-month administration
LUPRON DEPOT-PED for 6-month administration was evaluated in an open-label, multicenter, clinical study with 45 pediatric patients with central precocious puberty; 41 (91%) were females and 4 (9%) were males. The baseline age ranged from 4 to 10 years. There were 30 (67%) Caucasian; 7 (16%) Black; and 1 (2%) Asian.
Adverse reactions that occurred in ≥4% of all patients are shown in Table 4.
| Table 4. Adverse Reactions Occurring in ≥4% in Pediatric Patients with CPP
Receiving LUPRON DEPOT-PED for 6-month administration. |
|
| Total
(N = 45) n (%) |
|
| Injection Site Reactions a | 35 (78%) |
| Headache b | 15 (33%) |
| Psychiatric Events c | 10 (22%) |
| Abdominal Pain d | 8 (18%) |
| Diarrhea e | 7 (16%) |
| Hemorrhage f | 6 (13%) |
| Nausea and Vomiting | 6 (13%) |
| Pyrexia | 6 (13%) |
| Pruritus g | 5 (11%) |
| Pain in extremity | 4 (9%) |
| Rash | 3 (7%) |
| Back Pain | 3 (7%) |
| Ligament sprain | 3 (7%) |
| Weight increased | 3 (7%) |
| Fracture h | 2 (4%) |
| Breast tenderness i | 2 (4%) |
| Insomnia j | 2 (4%) |
| Chest pain | 2 (4%) |
| Hyperhidrosis | 2 (4%) |
| a Injection site reactions includes the preferred terms injection site pain, injection site erythema, injection site reaction, injection site warmth, injection site bruising, injection site discomfort, and injection site swelling b-Headache includes the preferred terms headache and cluster headache c Psychiatric events includes the preferred terms affect lability, affective disorder, aggression, crying, depressed mood, disruptive mood dysregulation disorder, hallucination auditory, mood altered, mood swings, and trichotillomania d Abdominal pain includes the preferred terms abdominal pain, abdominal pain upper, and abdominal discomfort e Diarrhea includes the preferred terms gastroenteritis and diarrhea f Hemorrhage includes the preferred terms contusion, epistaxis, hematochezia, and injection site bruising g Pruritus includes the preferred terms pruritus, vulvovaginal pruritus, nasal pruritus h Fracture includes the preferred terms ankle fracture and tibia fracture i Breast tenderness includes the preferred terms breast pain and breast tenderness j Insomnia includes the preferred terms initial insomnia and insomnia |
|
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of leuprolide acetate or LUPRON DEPOT-PED in pediatric patients. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Allergic reactions: anaphylactic, rash, urticaria, and photosensitivity reactions.
General: chest pain, weight increase, decreased appetite, fatigue.
Laboratory Abnormalities: decreased WBC.
Metabolic: diabetes mellitus.
Musculoskeletal and Connective Tissue: tenosynovitis-like symptoms, severe muscle pain, arthralgia, epiphysiolysis, muscle spasms, myalgia.
Published literature and postmarketing reports indicate that bone mineral density may decrease during GnRH therapy in pediatric patients with central precocious puberty. Published studies indicate that after discontinuation of therapy, subsequent bone mass accrual is preserved and peak bone mass in late adolescence does not seem to be affected.
Neurologic: neuropathy peripheral, convulsion, insomnia, pseudotumor cerebri (idiopathic intracranial hypertension).
Psychiatric Disorders: emotional lability, such as crying, irritability, impatience, anger, and aggression. Depression, including rare reports of suicidal ideation and attempt. Many, but not all, of these patients had a history of psychiatric illness or other comorbidities with an increased risk of depression.
Reproductive System: vaginal bleeding, breast enlargement.
Respiratory: dyspnea.
Skin and Subcutaneous Tissue: injection site reactions including induration and abscess, flushing, hyperhidrosis.
Vascular Disorders: hypertension, hypotension.
Related/similar drugs
7. Drug Interactions
7.1 Drug Interactions
No pharmacokinetic-based drug-drug interaction studies have been conducted with LUPRON DEPOT-PED [see Clinical Pharmacology (12.3)].
7.2 Drug-Laboratory Test Interactions
Administration of LUPRON DEPOT-PED in therapeutic doses results in suppression of the pituitary-gonadal system. Therefore, diagnostic tests of pituitary gonadotropic and gonadal functions conducted during treatment and up to six months after discontinuation of LUPRON DEPOT-PED may be affected. Normal pituitary-gonadal function is usually restored within six months after treatment with LUPRON DEPOT-PED is discontinued.
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
LUPRON DEPOT-PED is contraindicated in pregnancy [see Contraindications (4)].
LUPRON DEPOT-PED may cause fetal harm, when administered to a pregnant woman, based on findings from animal studies and the drug’s mechanism of action [see Clinical Pharmacology (12.1)]. The available data from published clinical studies and case reports and from the pharmacovigilance database on exposure to LUPRON DEPOT-PED during pregnancy are insufficient to assess the risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. Based on animal reproduction studies, LUPRON DEPOT-PED may be associated with an increased risk of pregnancy complications, including early pregnancy loss and fetal harm. In animal reproduction studies, subcutaneous administration of leuprolide acetate to rabbits during the period of organogenesis caused embryo-fetal toxicity, decreased fetal weights and a dose-dependent increase in major fetal abnormalities in animals at doses less than the recommended human dose based on body surface area using an estimated daily dose. A similar rat study also showed increased fetal mortality and decreased fetal weights but no major fetal abnormalities at doses less than the recommended human dose based on body surface area using an estimated daily dose (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the US general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% - 4% and 15% -20%, respectively.
Data
Animal Data
When administered on day 6 of pregnancy at test dosages of 0.00024 mg/kg, 0.0024 mg/kg, and 0.024 mg/kg (doses less than the recommended human dose) to rabbits, leuprolide acetate produced a dose-related increase in malformations comprised primarily of segmental and fusion defects of the skeleton and skull. Similar studies in rats failed to demonstrate an increase in fetal malformations. There was increased fetal mortality and decreased fetal weights with the two higher doses of leuprolide acetate in rabbits and with the highest dose (0.024 mg/kg) in rats.
8.2 Lactation
Risk Summary
There are no data on the presence of leuprolide acetate in either animal or human milk, the effects on the breastfed infants, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for LUPRON DEPOT-PED and any potential adverse effects on the breastfed infant from LUPRON DEPOT-PED or from the underlying maternal condition.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Exclude pregnancy in women of reproductive potential prior to initiating LUPRON DEPOT-PED if clinically indicated [see Use in Specific Populations (8.1)].
Contraception
Females
LUPRON DEPOT-PED may cause embryo-fetal harm when administered during pregnancy. LUPRON DEPOT-PED is not a contraceptive. If contraception is indicated, advise females of reproductive potential to use a non-hormonal method of contraception during treatment with LUPRON DEPOT-PED [see Use in Specific Populations (8.1)].
Infertility
Based on its pharmacodynamic effects of decreasing secretion of gonadal steroids, fertility is expected to be decreased while on treatment with LUPRON DEPOT-PED. Clinical and pharmacologic studies in adults (>18 years) with leuprolide acetate and similar analogs have shown reversibility of fertility suppression when the drug is discontinued after continuous administration for periods of up to 24 weeks [see Clinical Pharmacology (12.2)].
There is no evidence that pregnancy rates are affected following discontinuation of LUPRON DEPOT-PED.
Animal studies (prepubertal and adult rats and monkeys) with leuprolide acetate and other GnRH analogs have shown functional recovery of fertility suppression.
8.4 Pediatric Use
The safety and effectiveness of LUPRON DEPOT-PED for the treatment of CPP has been established in pediatric patients 1 years of age and older. Use of LUPRON DEPOT-PED for this indication is supported by evidence from two pivotal, open label clinical studies of 139 pediatric patients with central precocious puberty with an age range of 1 to 11 years [see Clinical Studies (14)]. The safety and effectiveness of LUPRON DEPOT-PED have not been established in pediatric patients less than 1 year old.
10. Overdosage
No specific antidotes for LUPRON DEPOT-PED are known. Contact Poison Control (1-800-222-1222) for latest recommendations.
In cases of overdosage, standard of care monitoring and management principles should be followed.
11. Lupron Depot-PED Description
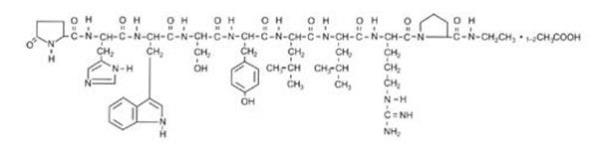
LUPRON DEPOT-PED contains active ingredient, leuprolide, in the form of acetate salt, a gonadotropin-releasing hormone (GnRH) agonist. It is a synthetic nonapeptide analog of naturally occurring gonadotropin-releasing hormone (GnRH or LH-RH). The analog possesses greater potency than the natural hormone. The chemical name of leuprolide acetate is 5-oxo-L-prolyl-L-histidyl-L-tryptophyl-L-seryl-L-tyrosyl-D-leucyl-L-leucyl-L-arginyl-N-ethyl-L-prolinamide acetate, which has molecular formula of C59H84N16O12.(C2H4O2)n, n=1 or 2, with the following structural formula:
LUPRON DEPOT-PED for 1-month administration
LUPRON DEPOT-PED is available in a prefilled dual-chamber single-dose syringe containing sterile lyophilized microsphere powder incorporated in a biodegradable lactic acid/glycolid acid copolymer which, when mixed with diluent, becomes a suspension for intramuscular injection. When mixed with 1 milliliter of accompanying diluent, LUPRON DEPOT-PED for 1-month administration is administered as a single-dose intramuscular injection.
The front chamber of LUPRON DEPOT-PED 7.5 mg, 11.25 mg, and 15 mg a prefilled dual-chamber syringe contains leuprolide acetate (7.5 mg equivalent to 6.83-7.15 mg leuprolide / 11.25 mg equivalent to 10.24 – 10.72 mg leuprolide / 15 mg equivalent to 13.65 – 14.30 mg leuprolide), purified gelatin (1.3/1.95/2.6 mg), DL-lactic and glycolic acids copolymer (66.2/99.3/132.4 mg), and D-mannitol (13.2/19.8/26.4 mg). The second chamber of diluent contains carboxymethylcellulose sodium (5 mg), D-mannitol (50 mg), polysorbate 80 (1 mg), water for injection, USP, and glacial acetic acid, USP to control pH.
LUPRON DEPOT-PED for 3-month administration
LUPRON DEPOT-PED 11.25 mg or 30 mg for 3-month administration is available in a prefilled dual-chamber single-dose syringe containing sterile lyophilized microsphere powder incorporated in a biodegradable lactic acid/glycolid acid copolymer which, when mixed with diluent, becomes a suspension for intramuscular injection. When mixed with 1.5 milliliters of accompanying diluent, LUPRON DEPOT-PED for 3-month administration is administered as a single-dose intramuscular injection.
The front chamber of LUPRON DEPOT-PED 11.25 mg for 3-month administration prefilled dual-chamber syringe contains leuprolide acetate (11.25 mg, equivalent to 10.24 - 10.72 mg leuprolide), D-mannitol (19.45 mg), and polylactic acid (99.3 mg). The second chamber of diluent contains carboxymethylcellulose sodium (7.5 mg), D-mannitol (75.0 mg), polysorbate 80 (1.5 mg), water for injection, USP, and glacial acetic acid, USP to control pH.
The front chamber of LUPRON DEPOT-PED 30 mg for 3-month administration prefilled dual-chamber syringe contains leuprolide acetate (30 mg, equivalent to 27.30 - 28.59 mg leuprolide), D-mannitol (51.9 mg), and polylactic acid (264.8 mg). The second chamber of diluent contains carboxymethylcellulose sodium (7.5 mg), D-mannitol (75.0 mg), polysorbate 80 (1.5 mg), water for injection, USP, and glacial acetic acid, USP to control pH.
LUPRON DEPOT-PED for 6-month administration
LUPRON DEPOT-PED 45 mg for 6-month administration is available in a prefilled dual-chamber syringe containing sterile lyophilized microspheres which, when mixed with diluent, become a suspension intended as an intramuscular injection.
The front chamber of LUPRON DEPOT-PED 45 mg for 6-month administration prefilled dual-chamber syringe contains leuprolide acetate (45 mg, equivalent to 40.95 - 42.89 mg leuprolide), D-mannitol (39.7 mg), polylactic acid (169.9 mg), and stearic acid (10.1 mg). The second chamber of diluent contains carboxymethylcellulose sodium (7.5 mg), D-mannitol (75.0 mg), polysorbate 80 (1.5 mg), water for injection, USP, and glacial acetic acid, USP to control pH.
12. Lupron Depot-PED - Clinical Pharmacology
12.1 Mechanism of Action
Leuprolide acetate, a GnRH agonist, acts as a potent inhibitor of gonadotropin secretion (LH and follicle stimulating hormone (FSH)) when given continuously in therapeutic doses.
12.2 Pharmacodynamics
Following an initial stimulation of GnRH receptors, chronic administration of leuprolide acetate results in downregulation of GnRH receptors, reduction in release of LH and FSH, and consequent suppression of ovarian and testicular production of estradiol and testosterone, respectively. This inhibitory effect is reversible upon discontinuation of drug therapy.
12.3 Pharmacokinetics
Absorption
LUPRON DEPOT-PED for 1-month administration
Following a single LUPRON DEPOT-PED 7.5 mg for 1-month administration to adult patients, mean peak leuprolide plasma concentration was almost 20 ng/mL at 4 hours and then declined to 0.36 ng/mL at 4 weeks. However, intact leuprolide and an inactive major metabolite could not be distinguished by the assay which was employed in the study. Nondetectable leuprolide plasma concentrations have been observed during chronic LUPRON DEPOT-PED 7.5 mg administration, but testosterone levels appear to be maintained at castrate levels.
In a study of pediatric patients with CPP, doses of 7.5 mg, 11.25 mg and 15.0 mg of LUPRON DEPOT-PED were given every 4 weeks. In 22 pediatric patients, trough leuprolide plasma levels were determined according to weight categories as summarized below:
| Patient Weight
Range (kg) | Group Weight
Average (kg) | Dose (mg) | Trough Plasma Leuprolide Level
Mean ±SD (ng/mL)* |
| 20.2 - 27.0 | 22.7 | 7.5 | 0.77±0.033 |
| 28.4 - 36.8 | 32.5 | 11.25 | 1.25±1.06 |
| 39.3 - 57.5 | 44.2 | 15.0 | 1.59±0.65 |
| * Group average values determined at Week 4 immediately prior to leuprolide injection. Drug levels at 12 and 24 weeks were similar to respective 4 week levels. | |||
LUPRON DEPOT-PED for 3-month administration
Following a single LUPRON DEPOT-PED 11.25 mg or 30 mg for 3-month administration to pediatric patients with CPP, leuprolide concentrations increased with increasing dose with mean peak leuprolide plasma concentration of 19.1 and 52.5 ng/mL at 1 hour for the 11.25 and 30 mg dose levels, respectively. The concentrations then declined to 0.08 and 0.25 ng/mL at 2 weeks after dosing for the 11.25 and 30 mg dose levels. Mean leuprolide plasma concentration remained constant from month 1 to month 3 for both 11.25 and 30 mg doses. The mean leuprolide concentrations 3 months after the first and second injections were similar indicating no accumulation of leuprolide from repeated administration.
LUPRON DEPOT-PED for 6-month administration
Following a single injection of LUPRON DEPOT-PED 45 mg for 6-month administration in 20 pediatric patients with CPP, mean peak plasma concentration increased rapidly to 15.7 ng/mL 1 hour post-dose. Following the initial rise, mean leuprolide plasma concentration declined to 0.03 ng/mL by Week 24. The mean leuprolide concentrations 6 months after the first and second injections were comparable indicating no accumulation of leuprolide from repeated administration.
Distribution
The mean steady-state volume of distribution of leuprolide following intravenous bolus administration to healthy male subjects was 27 L. In vitro binding to human plasma proteins ranged from 43% to 49%.
Elimination
Metabolism
In healthy male subjects given an intravenous 1 mg bolus of leuprolide the mean systemic clearance was 7.6 L/h, with a terminal elimination half-life of approximately 3 hours based on a two-compartment model.
In rats and dogs, administration of 14C-labeled leuprolide was shown to be metabolized to smaller inactive peptides; a pentapeptide (Metabolite I), tripeptides (Metabolites II and III) and a dipeptide (Metabolite IV). These fragments may be further catabolized.
The major metabolite (M-I) plasma concentrations measured in 5 prostate cancer patients reached maximum concentration 2 to 6 hours after dosing and were approximately 6% of the peak parent drug concentration. One week after dosing, mean plasma M-I concentrations were approximately 20% of mean leuprolide concentrations.
Excretion
Following administration of LUPRON DEPOT 3.75 mg to 3 patients, less than 5% of the dose was recovered as parent and M-I metabolite in the urine.
Specific Populations
The pharmacokinetics of LUPRON DEPOT-PED has not been determined in patients with hepatic or renal impairment.
Drug-Drug Interactions
No pharmacokinetic-based drug-drug interaction studies have been conducted with LUPRON DEPOT-PED. Leuprolide acetate is a peptide that is not degraded by cytochrome P-450 enzymes; hence, drug interactions associated with cytochrome P-450 enzymes would not be expected to occur.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
A two-year carcinogenicity study was conducted in rats and mice. In rats, a dose-related increase of benign pituitary hyperplasia and benign pituitary adenomas was noted at 24 months when the drug was administered subcutaneously at high daily doses (0.6 to 4 mg/kg). There was a significant but not dose-related increase of pancreatic islet-cell adenomas in females and of testicular interstitial cell adenomas in males (highest incidence in the low dose group). In mice, no leuprolide acetate-induced tumors or pituitary abnormalities were observed at a dose as high as 60 mg/kg for two years.
Mutagenicity studies have been performed with leuprolide acetate using bacterial and mammalian systems. These studies provided no evidence of a mutagenic potential.
14. Clinical Studies
14.1 LUPRON DEPOT-PED for 1-month administration
The efficacy of LUPRON DEPOT-PED was evaluated in a pivotal open-label, multicenter clinical trial (NCT00660010) in which 55 pediatric patients with central precocious puberty (49 females and 6 males, naïve to previous GnRHa treatment) were treated with LUPRON DEPOT-PED 1-month formulations until age was appropriate for entry into puberty (see treatment period data below)and a subset of 40 patients were then followed post-treatment (see follow-up period data below). The mean ± SD age at the start of treatment was 7 ± 2 years and the duration of treatment was 4 ± 2 years. Study drug was administered intramuscularly (IM) every 28 days, with incremental adjustments of 3.75mg at each clinic visit, if necessary based on clinical and laboratory results. During the follow-up period, GnRHa stimulation test was performed every 6 months until a pubertal response was observed.
During the treatment period, LUPRON DEPOT-PED suppressed gonadotropins and sex steroids to prepubertal levels. Suppression of peak stimulated LH concentrations to < 1.75 mIU/mL was achieved in 96% of patients by month 1. Five patients required increased doses of study drug to achieve or retain LH suppression. The number and percentage of patients with suppression of peak stimulated LH < 1.75 mIU/mL and mean ± SD peak stimulated LH over time is shown in Table 5. Six months after the treatment period was finished, the mean peak stimulated LH was 20.6 ± SD 13.7 mIU/mL (n=30).
The following effects have been noted with the chronic administration of leuprolide: cessation of menses (in girls), normalization and stabilization of linear growth and bone age advancement, stabilization of clinical signs and symptoms of puberty.
| Table 5. The number and percentage of patients with peak
stimulated LH < 1.75 mIU/mL and Mean (SD) peak LH at each clinic visit |
|||
| Weeks on Study | n with peak stimulated LH < 1.75 mIU/mL/
N with a LH measurement for that week | Mean (SD) peak LH | |
| n/N | % | ||
| Baseline | 0/55 | 0% | 35.0 (21.32) |
| Week 4 | 53/55 | 96.4% | 0.8 (0.57) |
| Week 12 | 48/54 | 88.9% | 1.1 (1.77) |
| Week 24 | 48/53 | 90.6% | 0.8 (0.79) |
| Week 36 | 51/54 | 94.4% | 0.6 (0.43) |
| Week 48 | 51/54 | 94.4% | 0.6 (0.47) |
| Week 72 | 52/52 | 100% | 0.5 (0.30) |
| Week 96 | 46/46 | 100% | 0.4 (0.33) |
| Week 120 | 40/40 | 100% | 0.4 (0.27) |
| Week 144 | 36/36 | 100% | 0.4 (0.24) |
| Week 168 | 27/28 | 96.4% | 1.2 (4.58) |
| Week 216 | 18/19 | 94.7% | 0.5 (0.90) |
| Week 240 | 16/17 | 94.1% | 0.4 (0.62) |
| Week 264 | 14/15 | 95.3% | 0.4 (0.41) |
| Week 288 | 11/11 | 100% | 0.3 (0.22) |
| Week 312 | 9/9 | 100% | 0.4 (0.20) |
| Week 336 | 6/6 | 100% | 0.3 (0.10) |
| Week 360 | 6/6 | 100% | 0.3 (0.13) |
| Week 384 | 5/5 | 100% | 0.2 (0.10) |
| Week 408 | 3/3 | 100% | 0.2 (0.09) |
| Week 432 | 2/2 | 100% | 0.3 (0.04) |
| Week 456 | 2/2 | 100% | 0.2 (0.04) |
| Week 480 | 1/1 | 100% | 0.2 (NA) |
| Week 504 | 1/1 | 100% | 0.2 (NA) |
Suppression (defined as regression or no change) of the clinical/physical signs of puberty was achieved in most patients. In females, suppression of breast development ranged from 66.7 to 90.6% of patients during the first 5 years of treatment. The mean stimulated estradiol was 15.1 pg/mL at baseline, decreased to the lower level of detection (5.0 pg/mL) by Week 4 and was maintained there during the first 5 years of treatment. In males, suppression of genitalia development ranged from 60% to 100% of patients during the first 5 years of treatment. The mean stimulated testosterone was 347.7 ng/dL at baseline and was maintained at levels no greater than 25.3 ng/dL during the first 5 years of treatment.
A “flare effect” of transient bleeding or spotting during the first 4 weeks of treatment was observed in 19.4% (7/36) females who had not reached menarche at baseline. After the first 4 weeks and for the remainder of the treatment period, no patients reported menstrual-like bleeding, and only rare spotting was noted.
The mean ratio of bone age to chronological age decreased from 1.5 at baseline to 1.1 by end of treatment. The mean height standard deviation z-score changed from 1.6 at baseline to 0.7 at the end of the treatment phase.
Thirty five (35) females and 5 males participated in a post-treatment follow-up period to assess reproductive function (in females) and final height. At 6 months post-treatment, most patients reverted to pubertal levels of LH (87.9%) and clinical signs of resumption of pubertal progression were evident with increase in breast development in girls (66.7%) and increase in genitalia development in boys (80%).
After stopping treatment, regular menses were reported for all female patients who reached 12 years of age during follow-up; mean time to menses was approximately 1.5 years; mean age of onset of menstruation after stopping treatment was 12.9 years.
Of the 40 patients evaluated in the follow-up, 33 were observed until they reached final or near-final adult height. These patients had a mean increase in final adult height compared to baseline predicted adult height. The mean final adult height standard deviation score was -0.2.
14.2 LUPRON DEPOT-PED for 3-month administration
The efficacy was evaluated in a 6-month, randomized, open-label clinical study of LUPRON DEPOT-PED 3-Month formulations (NCT00667446). 84 patients (76 female, 8 male) between 1 and 11 years of age received the LUPRON DEPOT-PED 11.25 mg or 30 mg for 3-month administration formulation. Each dose group had an equal number of treatment-naïve patients who had pubertal LH levels and patients previously treated with GnRHa therapies who had prepubertal LH levels at the time of study entry. The percentage of patients with suppression of peak-stimulated LH to < 4.0 mIU/mL, as determined by assessments at months 2, 3 and 6, is 78.6% in the 11.25 mg dose and 95.2% in the 30 mg dose as shown in Table 6.
| Table 6. Suppression of Peak-Stimulated LH from Month 2 Through Month 6 | ||||||
| LUPRON DEPOT-PED
11.25 mg every 3 Months | LUPRON DEPOT-PED
30 mg every 3 Months |
|||||
| Parameter | Naïve
N = 21 | Prev Trta
N = 21 | Total
N = 42 | Naïve
N = 21 | Prev Trta
N = 21 | Total
N = 42 |
| Percent with Suppression | 76.2 | 81.0 | 78.6 | 90.5 | 100 | 95.2 |
| 2-sided 95% CI | 52.8, 91.8 | 58.1, 94.6 | 63.2, 89.7 | 69.6, 98.8 | 83.9, 100 | 83.8, 99.4 |
a. Previously treated with GnRHa for at least 6 months prior to enrollment in pivotal Study L-CP07-167.
The mean peak stimulated LH levels for all visits are shown by dose and subgroup (naïve vs. previously treated patients) in Figures 8 and 9.
Figure 8. Mean Peak Stimulated LH for LUPRON DEPOT-PED 11.25 mg for 3-month administration
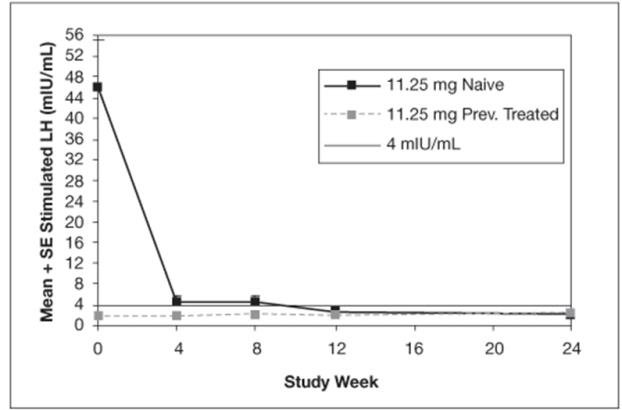
Figure 9. Mean Peak Stimulated LH for LUPRON DEPOT-PED 30 mg for 3-month administration
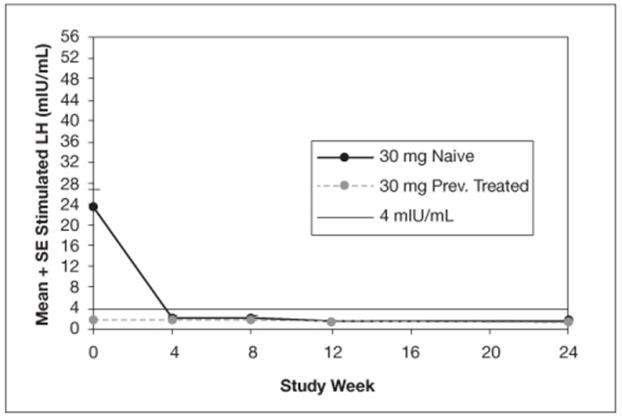
For the LUPRON DEPOT-PED 11.25 mg dose for 3-month administration, 93% (39/42) of patients and for LUPRON DEPOT-PED 30 mg dose for 3-month administration, 100% (42/42) of patients had sex steroid (estradiol or testosterone) suppressed to prepubertal levels at all visits. Clinical suppression of puberty in female patients was observed in 29 of 32 (90.6%) and 28 of 34 (82.4%) of patients in the 11.25 mg and 30 mg groups, respectively, at month 6. Clinical suppression of puberty in males was observed in 1 of 2 (50%) and 2 of 5 (40%) patients in the 11.25 mg and 30 mg groups, respectively, at month 6. In patients with complete data for bone age, 29 of 33 (88%) in the 11.25 mg group and 30 of 40 in the 30 mg group (75%) had a decrease in the ratio of bone age to chronological age at month 6 compared to screening.
14.3 LUPRON DEPOT-PED for 6-month administration
The safety and efficacy of LUPRON DEPOT-PED 6-Month formulation was evaluated in an open-label, single-arm, multicenter, clinical trial (NCT03695237).
In the clinical trial, 27 pediatric patients with central precocious puberty (24 females and 3 males) naïve to previous GnRH agonist treatment and 18 pediatric patients with central precocious puberty (17 females and 1 males) previously treated with GnRH agonist therapy (including 1-,3-, 6- and 12- month) received LUPRON DEPOT-PED 45 mg. LUPRON DEPOT-PED 45 mg was administered as an intramuscular injection at two intervals 24 weeks apart.
The primary efficacy endpoint of percentage of patients with suppression of peak-stimulated LH to < 4.0 mIU/mL at Week 24 was achieved in 39/45 (86.7%) patients (Table 7). Of the patients previously treated with other GnRHa formulations 94.4% remained suppressed at Week 24.
| Table 7. Suppression of Peak-Stimulated GnRHa-Stimulated LH at Week 24 | |||
| LUPRON DEPOT-PED
45 mg |
|||
| Parameter | Naïve
N = 27 | Prev Trta
N = 18 | Total
N =45 |
| Percent with Suppression, n (%) | 22 (81.5) | 17 (94.4) | 39 (86.7) |
| 95% CI | 61.9, 93.7 | 72.7, 99.9 | 73.2, 95.0 |
- Previously treated with GnRHa for at least 6 months prior to enrollment in pivotal Study M16-904
The mean peak stimulated LH levels decreased from 17.4 mIU/mL in treatment-naïve patients and from 2.1 mIU/ML in previously treated patients at baseline to 1.6 mIU/mL and 1.5 mIU/mL respectively at Week 4. The levels remained suppressed for all visits up to Week 48.
The proportion of female patients with suppression of basal estradiol to <20 pg/mL was 97.4% at Week 24 and 100% at Week 48. The proportion of male patients with suppression of testosterone to <30 ng/dL was 100% at Weeks 24 and 48. Suppression (defined as regression or no change) of the physical signs of puberty was achieved in most patients.
In patients with complete data for bone age, 40 of 45 (88.9%) and 42 of 45 (93.3%) had a decrease in the ratio of bone age to chronological age at Weeks 24 and 48, respectively, compared to baseline.
In the extension part of the study (Weeks 49 to 144), 4 of 45 enrolled patients discontinued due to lack of efficacy. Twenty-three patients had complete data available through Week 144; LH suppression was maintained in all 23 patients.
16. How is Lupron Depot-PED supplied
How Supplied
LUPRON DEPOT-PED for depot suspension is supplied in a single dose, prefilled dual-chamber syringe containing a white lyophilized powder and a colorless diluent for reconstitution as follows (Table 8):
| LUPRON DEPOT-PED 7.5 mg, 11.25 mg, or 15 mg for 1-Month Administration | ||
| Kit Type | Strength | NDC Number |
| 1-month kit | 7.5 mg | NDC 0074-2108-03 |
| 11.25 mg | NDC 0074-2282-03 | |
| 15 mg | NDC 0074-2440-03 | |
| LUPRON DEPOT-PED 11.25 mg or 30 mg for 3-Month Administration | ||
| 3-month kit | 11.25 mg | NDC 0074-3779-03 |
| 30 mg | NDC 0074-9694-03 | |
| LUPRON DEPOT-PED 45 mg for 6-Month Administration | ||
| 6-month kit | 45 mg | NDC 0074-3575-01 |
Each kit contains:
- one single-dose, prefilled dual-chamber syringe containing 23 gauge 1½ inch needle with LuproLoc® safety device
- one plunger
- two alcohol swabs
- population, dose and frequency confirmation insert
- a complete prescribing information enclosure
Storage and Handling
Prior to reconstitution, store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [See USP Controlled Room Temperature].
After reconstitution, use immediately [see Dosage and Administration (2.5, 2.6)].
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Symptoms After Initial LUPRON DEPOT-PED Administration
Inform patients and caregivers that during the early phase of therapy with LUPRON DEPOT-PED, gonadotropins and sex steroids rise above baseline because of the initial stimulatory effect of the drug. Therefore, an increase in clinical signs and symptoms of puberty may be observed. Instruct patients and caregivers to notify the physician if these symptoms continue beyond the second month after LUPRON DEPOT-PED administration [see Warnings and Precautions (5.1)].
Psychiatric Events
Inform patients and caregivers that psychiatric events have been reported in patients taking GnRH agonists, including leuprolide acetate. Events include emotional lability, such as crying, irritability, impatience, anger, and aggression. Instruct patients and caregivers to monitor for development or worsening of psychiatric symptoms including depression during treatment with LUPRON DEPOT-PED [see Warnings and Precautions (5.2)].
Convulsions
Inform patients and caregivers that reports of convulsions have been observed in patients receiving GnRH agonists, including leuprolide acetate. Patients with a history of seizures, epilepsy, cerebrovascular disorders, central nervous system anomalies or tumors, and patients on concomitant medications that have been associated with convulsions may be at increased risk [see Warnings and Precautions (5.3)].
Pseudotumor Cerebri (Idiopathic Intracranial Hypertension)
Inform patients and caregivers that reports of pseudotumor cerebri (idiopathic intracranial hypertension) have been observed in pediatric patients receiving GnRH agonists, including LUPRON DEPOT-PED. Advise patients and caregivers to monitor for headache and vision issues such as blurred vision, double vision, loss of vision, pain behind the eye or pain with eye movement, ringing in the ears, dizziness, and nausea. Advise patients and caregivers to contact their healthcare provider if the patient develops any of these symptoms [see Warnings and Precautions (5.4)].
Injection Site Reactions
Inform patients and caregivers that injection site related adverse reactions may occur such as transient burning/stinging, pain, bruising, and redness. Advise patients to contact their healthcare provider if they experience rash or severe injection site reactions [see Adverse Reactions (6.1)].
Pregnancy
LUPRON DEPOT-PED is contraindicated in pregnancy. If the patient becomes pregnant while taking the drug, the patient should be informed of the potential risk to the fetus [see Use in Specific Populations (8.1)].
Compliance with the Dosing Schedule
Inform caregivers about the importance of adherence to the LUPRON DEPOT-PED dosing schedule [see Dosage and Administration (2.2, 2.3, 2.4)].
Manufactured for
AbbVie Inc.
North Chicago, IL 60064
by Takeda Pharmaceutical Company Limited
Osaka, Japan 540-8645
20093338
Medication Guide
| MEDICATION GUIDE
LUPRON DEPOT-PED® (loo-pron depo peed) (leuprolide acetate for depot suspension) |
||||
What is the most important information I should know about LUPRON DEPOT-PED?
|
||||
| ◦ headache ◦ eye problems, including blurred vision, double vision and decreased eyesight ◦ eye pain | ◦ ringing in the ears ◦ dizziness ◦ nausea |
|||
What is LUPRON DEPOT-PED?
|
||||
LUPRON DEPOT-PED should not be taken if your child is:
|
||||
Before your child receives LUPRON DEPOT-PED, tell their doctor about all of your child’s medical conditions including if they:
|
||||
How will your child receive LUPRON DEPOT-PED?
|
||||
| What are the possible side effects of LUPRON DEPOT-PED?
LUPRON DEPOT-PED may cause serious side effects. See “What is the most important information I should know about LUPRON DEPOT-PED?” The most common side effects of LUPRON DEPOT-PED received 1 time each month include:
|
||||
|
|
|
||
|
These are not all the possible side effects of LUPRON DEPOT-PED. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
||||
How should I store LUPRON DEPOT-PED INJECTION?
|
||||
| General information about the safe and effective use of LUPRON DEPOT-PED.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use LUPRON DEPOT-PED for a condition for which it was not prescribed. This Medication Guide summarizes the most important information about LUPRON DEPOT-PED. If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about LUPRON DEPOT-PED that is written for doctors or trained nurses. |
||||
| What are the ingredients in LUPRON DEPOT-PED?
LUPRON DEPOT-PED 7.5 mg, 11.25 mg or 15 mg for 1-month administration: Active Ingredients: leuprolide acetate for depot suspension Inactive Ingredients: purified gelatin, DL-lactic and glycolic acids copolymer, D-mannitol, carboxymethylcellulose sodium, polysorbate 80, water for injection, USP, and glacial acetic acid, USP to control pH. LUPRON DEPOT-PED 11.25 mg or 30 mg for 3-month administration: Active Ingredients: leuprolide acetate for depot suspension Inactive Ingredients: polylactic acid, D-mannitol, carboxymethylcellulose sodium, polysorbate 80, water for injection, USP, and glacial acetic acid, USP to control pH. LUPRON DEPOT-PED 45 mg for 6-month administration: Active Ingredients: leuprolide acetate for depot suspension Inactive Ingredients: polylactic acid, D-mannitol, and stearic acid, carboxymethylcellulose sodium, D-mannitol, polysorbate 80, water for injection, USP, and glacial acetic acid, USP to control pH. Manufactured for: AbbVie Inc. North Chicago, IL 60064 By Takeda Pharmaceutical Company Limited Osaka, Japan 540-8645 For more information, go to www.lupronped.com or call 1-800-633-9110. |
||||
| This Medication Guide has been approved by the U.S. Food and Drug Administration. | Revised: May 2025 | |||
20093338
NDC 0074-2108-03
PEDIATRIC USE ONLY 7.5 mg for 1-month administration
Single Dose Administration Kit with prefilled dual-chamber syringe.
LUPRON DEPOT-PED®
(Leuprolide Acetate for Depot Suspension)
Dispense the accompanying Medication Guide to each patient.
7.5 mg for 1-month administration
FOR INTRAMUSCULAR INJECTION
The front chamber contains: leuprolide acetate 7.5 mg۰purified gelatin 1.3 mg۰DL-lactic & glycolic acids copolymer 66.2 mg۰D-mannitol 13.2 mg
The second chamber contains: D-mannitol 50 mg۰ carboxymethylcellulose sodium 5 mg۰polysorbate 80 1 mg۰water for injection, USP and glacial acetic acid, USP to control pH
Rx only

NDC 0074-2282-03
PEDIATRIC USE ONLY 11.25 mg for 1-month administration
Single Dose Administration Kit with prefilled dual-chamber syringe.
LUPRON DEPOT-PED®
(Leuprolide Acetate for Depot Suspension)
Dispense the accompanying Medication Guide to each patient.
11.25 mg for 1-month administration
FOR INTRAMUSCULAR INJECTION
The front chamber contains: leuprolide acetate 11.25 mg۰purified gelatin 1.95 mg۰DL-lactic & glycolic acids copolymer 99.3 mg۰D-mannitol 19.8 mg
The second chamber contains: D-mannitol 50 mg۰carboxymethylcellulose sodium 5 mg۰polysorbate 80 1 mg۰water for injection, USP, and glacial acetic acid, USP to control pH
Rx only
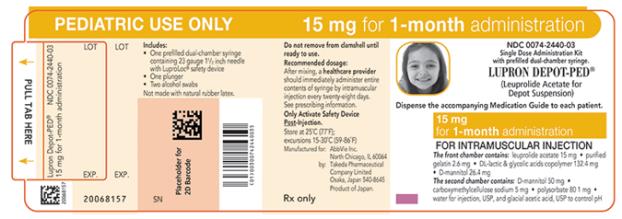
NDC 0074-2440-03
PEDIATRIC USE ONLY 15 mg for 1-month administration
Single Dose Administration Kit with prefilled dual-chamber syringe.
LUPRON DEPOT-PED®
(Leuprolide Acetate for Depot Suspension)
Dispense the accompanying Medication Guide to each patient.
15 mg for 1-month administration
FOR INTRAMUSCULAR INJECTION
The front chamber contains: leuprolide acetate 15 mg۰purified gelatin 2.6 mg۰DL-lactic & glycolic acids copolymer 132.4 mg۰D-mannitol 26.4 mg
The second chamber contains: D-mannitol 50 mg۰ carboxymethylcellulose sodium 5 mg۰polysorbate 80 1 mg۰water for injection, USP and glacial acetic acid, USP to control pH
Rx only
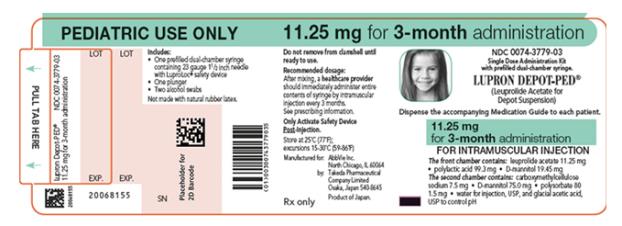
NDC 0074-3779-03
PEDIATRIC USE ONLY 11.25 mg for 3-month administration
Single Dose Administration Kit with prefilled dual-chamber syringe.
LUPRON DEPOT-PED® (Leuprolide Acetate for Depot Suspension)
Dispense the accompanying Medication Guide to each patient.
11.25 mg for 3-month administration
FOR INTRAMUSCULAR INJECTION
The front chamber contains: leuprolide acetate 11.25 mg۰polylactic acid 99.3 mg۰D-mannitol 19.45 mg
The second chamber contains: carboxymethylcellulose sodium 7.5 mg۰D-mannitol 75.0 mg۰polysorbate 80 1.5 mg۰water for injection, USP, and glacial acetic acid, USP to control pH
Rx only
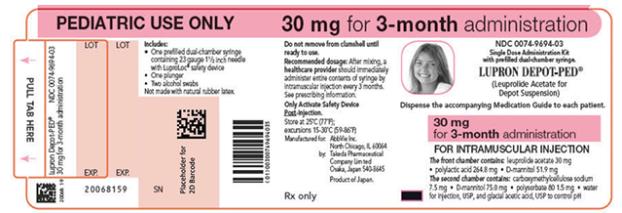
NDC 0074-9694-03
PEDIATRIC USE ONLY 30 mg for 3-month administration
Single Dose Administration Kit with prefilled dual-chamber syringe
LUPRON DEPOT-PED®
(Leuprolide Acetate for Depot Suspension)
Dispense the accompanying Medication Guide to each patient.
30 mg for 3-month administration
FOR INTRAMUSCULAR INJECTION
The front chamber contains: leuprolide acetate 30 mg۰polylactic acid 264.8 mg۰D-mannitol 51.9 mg
The second chamber contains: carboxymethylcellulose sodium 7.5 mg۰D-mannitol 75.0 mg۰polysorbate 80 1.5 mg۰water for injection, USP, and glacial acetic acid, USP to control pH
Rx only
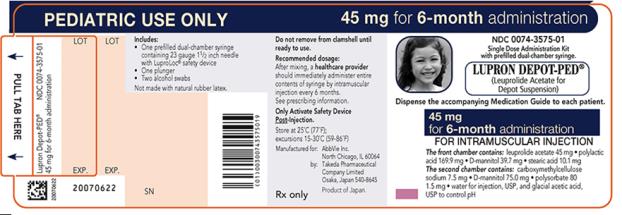
NDC 0074-3575-01
PEDIATRIC USE ONLY 45 mg for 6-month administration
Single Dose Administration Kit with prefilled dual-chamber syringe
LUPRON DEPOT-PED®
(Leuprolide Acetate for Depot Suspension)
Dispense the accompanying Medication Guide to each patient.
45 mg for 6-month administration
FOR INTRAMUSCULAR INJECTION
The front chamber contains: leuprolide acetate 45 mg۰polylactic acid 169.9 mg۰D-mannitol 39.7 mg۰stearic acid 10.1 mg
The second chamber contains: carboxymethylcellulose sodium 7.5 mg۰D-mannitol 75.0 mg۰polysorbate 80 1.5 mg۰water for injection, USP, and glacial acetic acid, USP to control pH
Rx only
| LUPRON DEPOT-PED
leuprolide acetate kit |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| LUPRON DEPOT-PED
leuprolide acetate kit |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| LUPRON DEPOT-PED
leuprolide acetate kit |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| LUPRON DEPOT-PED
leuprolide acetate kit |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| LUPRON DEPOT-PED
leuprolide acetate kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| LUPRON DEPOT-PED
leuprolide acetate kit |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - AbbVie Inc. (078458370) |
Frequently asked questions
- Are Lupron Depot and Eligard the same drug?
- Will I get my period while on Lupron?
- What does Lupron do for IVF?
- Can you get pregnant on Lupron Depot?
- Is Firmagon (degarelix) the same as Lupron Depot?
More about Lupron Depot-PED (leuprolide)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (25)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Drug class: gonadotropin releasing hormones
- En español
Professional resources
Other brands
Eligard, Camcevi, Lupron Depot-Gyn, Fensolvi, Lutrate Depot