Camcevi: Package Insert / Prescribing Info
Package insert / product label
Generic name: leuprolide
Dosage form: injection, emulsion
Drug classes: Gonadotropin releasing hormones, Hormones / antineoplastics
J Code (medical billing code): J1952 (Leuprolide inj, camcevi, 1mg)
Medically reviewed by Drugs.com. Last updated on May 26, 2025.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Use In Specific Populations
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
CAMCEVI (leuprolide) injectable emulsion, for subcutaneous use
Initial U.S. Approval: 2021
Recent Major Changes
Dosage and Administration ( 2.2) 02/2025
Indications and Usage for Camcevi
CAMCEVI is a gonadotropin-releasing hormone (GnRH) agonist indicated for the treatment of adult patients with advanced prostate cancer. (1)
Camcevi Dosage and Administration
Dosage Forms and Strengths
- Injectable emulsion: 42 mg ( 3)
Contraindications
- Hypersensitivity to GnRH, GnRH agonist analogs, or any of the components of CAMCEVI. ( 4)
Warnings and Precautions
- Tumor Flare: Transient worsening of bone pain, uretral obstruction, spinal cord compression, or the occurrence of additional signs and symptoms of prostate cancer may develop during the first few weeks of treatment. Monitor patients closely and manage symptoms. ( 5.1)
- Hyperglycemia and Diabetes: Hyperglycemia and an increased risk of developing diabetes have been reported in men receiving GnRH agonists. Monitor blood glucose levels and manage according to current clinical practice. ( 5.2)
- Cardiovascular Diseases: Increased risk of myocardial infarction, sudden cardiac death, and stroke has been reported in men receiving GnRH agonists. Monitor for cardiovascular disease and manage according to current clinical practice. ( 5.3)
- QT/QTc Prolongation: Androgen deprivation therapy may prolong the QT interval. Consider periodic monitoring of electrocardiograms and electrolytes. ( 5.4)
- Convulsions: Manage convulsions according to the current clinical practice. ( 5.5)
- Embryo-Fetal Toxicity: CAMCEVI may cause fetal harm. ( 5.7, 8.1)
Adverse Reactions/Side Effects
The most common (≥5%) adverse reactions were hot flushes, hypertension, injection site reactions, fatigue, upper respiratory tract infections, musculoskeletal pain, pain in extremity, arthralgia, micturition urgency, nocturia, and dizziness. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Accord BioPharma Inc. at 1-866-941-7875 or FDA at 1-800-FDA-1088 orwww.fda.gov/medwatch
Use In Specific Populations
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 2/2025
Full Prescribing Information
1. Indications and Usage for Camcevi
CAMCEVI is indicated for the treatment of adult patients with advanced prostate cancer.
2. Camcevi Dosage and Administration
2.1 Recommended Dosage
The recommended dose of CAMCEVI is 42 mg administered subcutaneously once every 6 months.
2.2 Preparation and Administration
CAMCEVI must be administered by a healthcare provider.
Important:Read the instructions completely before you administer Camcevi for the first time. Do NOT substitute any of the components from the kit for administration.
CAMCEVI is packaged in a blister in the kit. Check to make sure the kit contains:
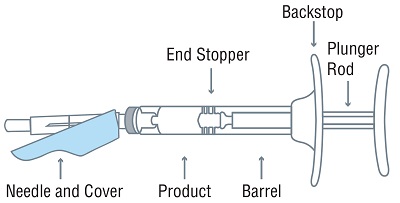
- One sterile, single-dose pre-filled syringe with plunger rod and backstop
- One sterile 18-gauge SurGuard®3 safety needle, 5/8-inch needle
- Prescribing Information
Follow the detailed instructions to ensure correct preparation of CAMCEVI prior to administration:
| STEP 1
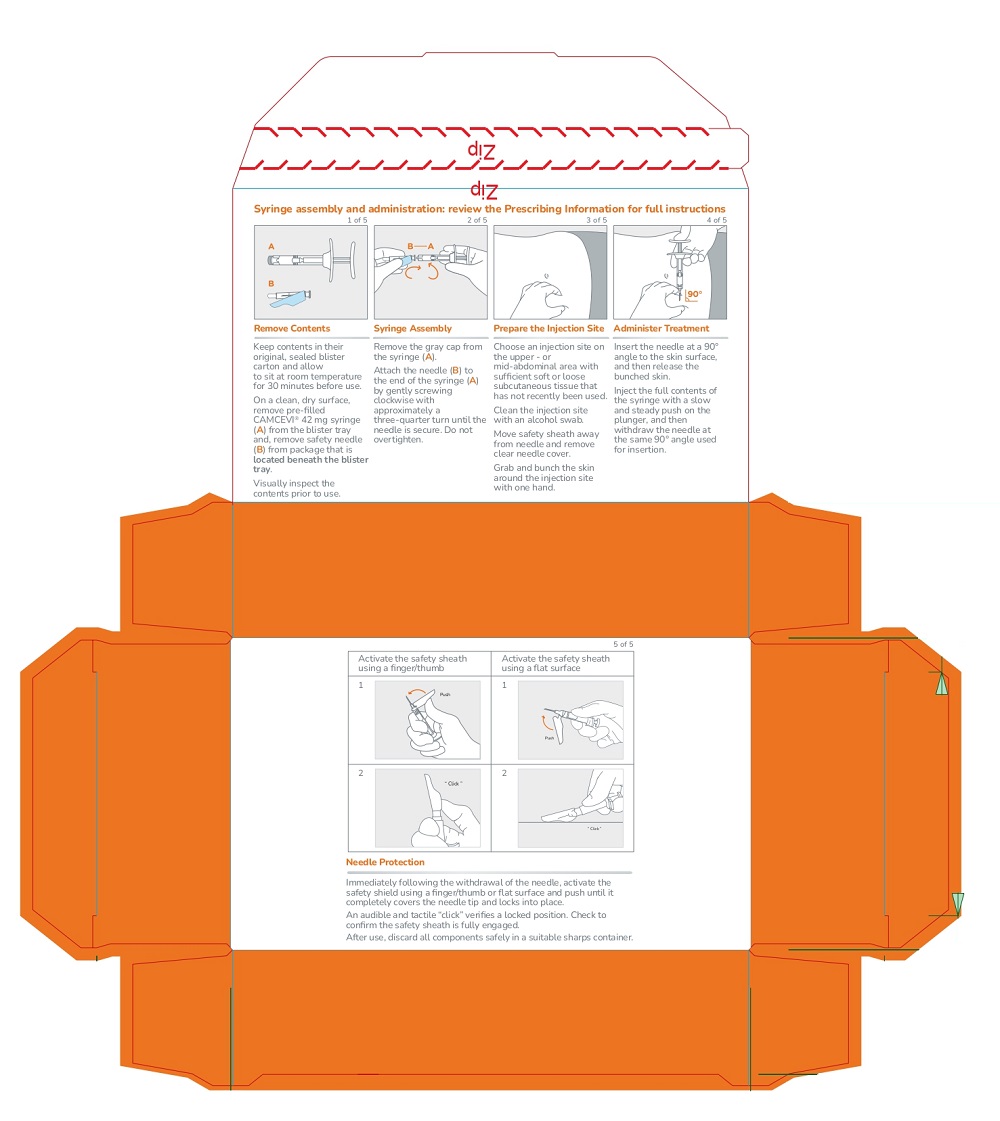
Remove CAMCEVI kit from refrigerator. Keep the contents in their original, sealed blister carton and allow to sit at room temperature for 30 minutes before use. Return to refrigerator after 30 minutes if not used. |
||
 | STEP 2
On a clean, dry surface, open carton and remove the contents. Examine all contents of the package. Do not use if any component is damaged. Check the expiration date on the syringe. Do not use if the expiration date has passed. The use of gloves is recommended during syringe assembly and administration. |
|
 | STEP 3
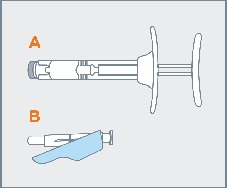
Remove pre-filled syringe (A) from the blister tray and open the safety needle (B) package by peeling back the paper tab. The safety needle (B) package is located beneath the blister tray. Visually inspect the syringe for particulate matter prior to administration. The emulsion should appear off-white to pale yellow, viscous, and opalescent. Do not use if particulate matter is observed prior to administration. |
|
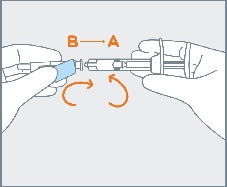 |
STEP 4
|
|
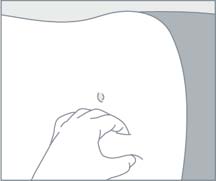 | STEP 5
Choose an injection site on the upper- or mid-abdominal area with sufficient soft or loose subcutaneous tissue that has not recently been used. Clean the injection site with an alcohol swab. Do NOTinject in areas with brawny or fibrous subcutaneous tissue or locations that can be rubbed or compressed (i.e., with a belt or clothing waistband). In addition, avoid applying heat directly to the site of Camcevi injection. |
|
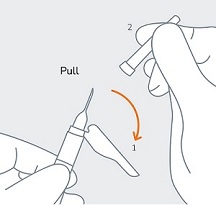 | STEP 6
(1) Move the safety sheath away from the needle and towards the syringe and (2) remove the clear needle cover immediately before injection. Note:Should the needle hub appear to be damaged, or leak, the product should NOT be used. The damaged needle should NOT be replaced and the product should NOT be injected. In the event of damage to the needle hub, use a new replacement CAMCEVI kit. |
|
 | STEP 7
Use standard aseptic technique when performing the injection. Grab and bunch the skin around the injection site with one hand. Insert the needle at a 90° angle to the skin surface, and then release the bunched skin. |
|
| STEP 8
Inject the full contents of the syringe with a slow and steady push on the plunger, and then withdraw the needle at the same 90° angle used for insertion. |
||
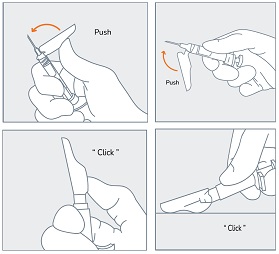 | STEP 9
Immediately following the withdrawal of the needle, activate the safety sheath using a finger/thumb or flat surface and push until it completely covers the needle tip and locks into place. An audible and tactile “click” verifies a locked position. Check to confirm the safety sheath is fully engaged. |
|
| STEP 10
After use, discard all components safely in a suitable sharps container. Dispose of the syringe and contaminated products according to local regulations/procedures. |
||
3. Dosage Forms and Strengths
Injectable emulsion: 42 mg leuprolide (equivalent to approximately 48 mg leuprolide mesylate) as a sterile, off-white to pale yellow, viscous, and opalescent emulsion in a single-dose, pre-filled syringe for subcutaneous injection.
4. Contraindications
CAMCEVI is contraindicated in patients known to be hypersensitive to GnRH, GnRH agonist analogs, or any of the excipients in CAMCEVI. Anaphylactic reactions to GnRH agonist analogs have been reported in the medical literature.
5. Warnings and Precautions
5.1 Tumor Flare
CAMCEVI, like other GnRH agonists, causes a transient increase in serum levels of testosterone during the first week of treatment, declining thereafter to baseline levels or below by the end of the second week of treatment. Transient worsening of symptoms, or the occurrence of additional signs and symptoms of prostate cancer, may develop during the first few weeks of CAMCEVI treatment. Patients treated with CAMCEVI may experience a temporary increase in bone pain, which can be managed symptomatically.
Cases of ureteral obstruction and spinal cord compression have been observed, which may contribute to paralysis with or without fatal complications.
Patients with metastatic vertebral lesions and/or with urinary tract obstruction should be closely observed during the first few weeks of therapy.
5.2 Hyperglycemia and Diabetes
Hyperglycemia and an increased risk of developing diabetes have been reported in men receiving GnRH agonists. Hyperglycemia may represent the development of diabetes mellitus or worsening of glycemic control in patients with diabetes. Monitor blood glucose and/or glycosylated hemoglobin (HbA1c) periodically in patients receiving a GnRH agonist and manage with current practice for treatment of hyperglycemia or diabetes.
5.3 Cardiovascular Diseases
Increased risk of developing myocardial infarction, sudden cardiac death, and stroke has been reported in association with use of GnRH agonists in men. The risk appears low based on the reported odds ratios, and should be evaluated carefully along with cardiovascular risk factors when determining a treatment for patients with prostate cancer. Patients receiving a GnRH agonist should be monitored for symptoms and signs suggestive of development of cardiovascular disease and be managed according to current clinical practice.
5.4 QT/QTc Prolongation
Androgen deprivation therapy may prolong the QT/QTc interval. Providers should consider whether the benefits of androgen deprivation therapy outweigh the potential risks in patients with congenital long QT syndrome, congestive heart failure, frequent electrolyte abnormalities, and in patients taking drugs known to prolong the QT interval. Electrolyte abnormalities should be corrected. Consider periodic monitoring of electrocardiograms and electrolytes.
5.5 Convulsions
Convulsions have been reported in patients receiving GnRH agonists, like CAMCEVI [see Adverse Reactions ( 6.2)]. Manage patients receiving a GnRH agonist who experience convulsions according to current clinical practice.
5.6 Laboratory Tests
Monitor serum levels of testosterone following injection of CAMCEVI. In the majority of patients treated with CAMCEVI, testosterone levels increased above baseline during the first week, and then declined thereafter to castration levels (<50 ng/dL) within 4 weeks [see Clinical Studies ( 14) and Adverse Reactions ( 6)].
5.7 Embryo-Fetal Toxicity
Based on findings in animal studies and mechanism of action, CAMCEVI can cause fetal harm when administered to a pregnant woman. In animal developmental and reproductive toxicology studies, administration of a monthly formulation of leuprolide on day 6 of pregnancy (sustained exposure was expected throughout the period of organogenesis) caused adverse embryo-fetal toxicity in animals at doses less than the human dose based on body surface area using an estimated daily dose. Advise pregnant patients and females of reproductive potential of the potential risk to the fetus [see Use in Specific Population ( 8.1), Clinical Pharmacology ( 12.1)].
6. Adverse Reactions/Side Effects
The following adverse reactions are discussed in greater detail in other sections of the labeling:
- Tumor Flare [see Warnings and Precautions (5.1)]
- Hyperglycemia and Diabetes [see Warnings and Precautions (5.2)]
- Cardiovascular Diseases [see Warnings and Precautions (5.3)]
- QT/QTc Prolongation [see Warnings and Precautions (5.4)]
- Convulsions [see Warnings and Precautions (5.5)]
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
FP01C-13-001
The safety of CAMCEVI was evaluated in an open-label, single-arm, international clinical trial (FP01C-13-001) in patients with advanced prostate cancer. Patients received CAMCEVI administered subcutaneously at a dose of 42 mg on Day 0 and Day 168. Of 137 patients enrolled, 93% received both doses of CAMCEVI.
Serious adverse reactions occurred in 15% of patients who received CAMCEVI, including 1% of patients who experienced subdural hematoma. Fatal adverse reactions occurred in 2% of patients, including cerebrovascular accident (0.7%) and pulmonary embolism (0.7%).
The most common adverse reactions (≥10%) occurring during a median follow-up duration of 336 days were hot flush, hypertension, injection site reactions, upper respiratory tract infections, musculoskeletal pain, fatigue, and pain in extremity.
Table 1 summarizes the adverse reactions in FP01C-13-001.
Table 1. Adverse Reactions Occurring in ≥5% of CAMCEVI in Advanced Prostate Cancer Patients - FP01C-13-001
| Adverse Reaction | CAMCEVI
N=137 |
|
| All Grades
(%) | Grade 3-4
(%) |
|
| Vascular disorders | ||
| Hot flushes a | 50 | 0 |
| Hypertension b | 15 | 0 |
| General disorders and administration site conditions | ||
| Injection site reactions c | 11 | 0 |
| Fatigue d | 10 | 0 |
| Infections and infestations | ||
| Upper respiratory tract infection e | 11 | 0 |
| Musculoskeletal and connective tissue disorders | ||
| Musculoskeletal pain f | 11 | 0 |
| Pain in extremity | 10 | 0 |
| Arthralgia | 7 | 0 |
| Renal and urinary disorders | ||
| Micturition urgency g | 6 | 0 |
| Nocturia | 6 | 0 |
| Nervous system disorders | ||
| Dizziness h | 5 | 0.7 |
aincludes hot flush and flushing
bincludes hypertension, essential hypertension, and blood pressure increased
cincludes injection site pain, injection site erythema, injection site hemorrhage, injection site nodule, injection site paraesthesia, injection site pruritus, and injection site warmth
dincludes fatigue and asthenia
eincludes upper respiratory tract infection, sinusitis, and nasopharyngitis
fincludes musculoskeletal pain, back pain, and bone pain
gincludes micturition urgency and dysuria
hincludes dizziness, dizziness postural, vertigo, and vertigo positional.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of CAMCEVI or leuprolide. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
During postmarketing surveillance, which includes other dosage forms and other patient populations, the following adverse reactions were reported.
Allergic Conditions: hypersensitivity reactions including anaphylaxis, rash, urticaria, and photosensitivity reactions
Cardiovascular System:hypotension, myocardial infarction, pulmonary embolism
Central/Peripheral Nervous System:convulsion, peripheral neuropathy, spinal fracture/paralysis
Endocrine System:pituitary apoplexy, diabetes
Hepato-biliary disorder:drug-induced liver injury
Hematologic:leukopenia
Psychiatric:mood swings, including depression, suicidal ideation and attempt
Respiratory, thoracic and mediastinal disorder:interstitial lung disease
Musculoskeletal System:decreased bone density, tenosynovitis-like symptoms, fibromyalgia
Skin and Subcutaneous:injection site induration or swelling
Urogenital System:prostate pain
Related/similar drugs
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
Based on findings in animal studies and mechanism of action, CAMCEVI can cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology ( 12.1)]. There are no available data in pregnant women to inform the drug-associated risk. In animal developmental and reproductive toxicology studies, administration of a monthly formulation of leuprolide on day 6 of pregnancy (sustained exposure was expected throughout the period of organogenesis) caused adverse embryo-fetal toxicity in animals at doses less than the human dose based on body surface area using an estimated daily dose (see data). Advise pregnant patients and females of reproductive potential of the potential risk to the fetus.
Data
Animal Data
Major fetal malformations were observed in developmental and reproductive toxicology studies in rabbits after a single administration of a monthly formulation of leuprolide administered on day 6 of pregnancy at test dosages of 0.00024, 0.0024, and 0.024 mg/kg (approximately 1/1500 to 1/15 the human dose based on body surface area using an estimated daily dose in animals and humans). Since a depot formulation was utilized in the study, a sustained exposure to leuprolide was expected throughout the period of organogenesis and to the end of gestation. Similar studies in rats did not demonstrate an increase in fetal malformations, however, there was increased fetal mortality and decreased fetal weights with the two higher doses of the monthly formulation of leuprolide in rabbits and with the highest dose in rats.
8.2 Lactation
Risk Summary
The safety and efficacy of CAMCEVI have not been established in females. There is no information regarding the presence of leuprolide in human milk, the effects on the breastfed child, or the effects on milk production. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in a breastfed child, a decision should be made to discontinue breastfeeding or discontinue the drug, taking into account the importance of the drug to the mother.
8.3 Females and Males of Reproductive Potential
Infertility
Males
Based on findings in animals and mechanism of action, CAMCEVI may impair fertility in males of reproductive potential [seeClinical Pharmacology ( 12.1), Nonclinical Toxicology ( 13.1) ].
11. Camcevi Description
CAMCEVI is a sterile formulation of leuprolide mesylate for subcutaneous injection. CAMCEVI is designed to deliver approximately 42 mg of leuprolide over 6 months.
Leuprolide mesylate is a synthetic nonapeptide analog of naturally occurring GnRH and is a GnRH agonist. The analog possesses greater potency than the natural hormone. The chemical name is 5-oxo-L-prolyl-L-histidyl-L-tryptophyl-L-seryl-L-tyrosyl-D-leucyl-L-leucyl-L-arginyl-N-ethyl-L-prolinamide mesylate (salt) with the following structural formula. The pH of 50 mg/mL solution of leuprolide mesylate in water is approximately 5.7.
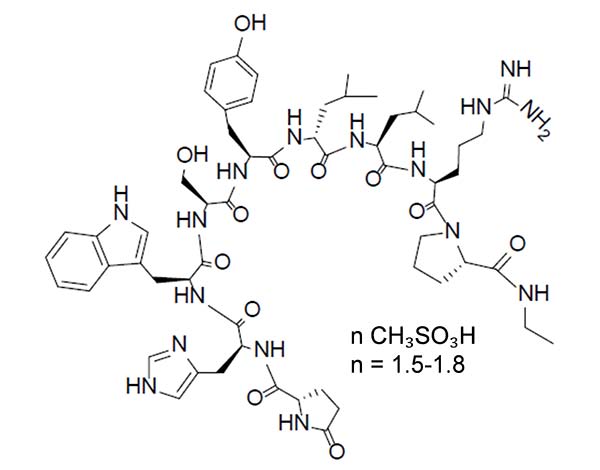
CAMCEVI is supplied as a kit with a pre-filled, single-dose, sterile syringe for subcutaneous injection. Each pre-filled syringe delivers 42 mg leuprolide (equivalent to approximately 48 mg leuprolide mesylate), poly(D, L-lactide) (184 mg) polymer and N-methyl-2-pyrrolidone (136 mg).
12. Camcevi - Clinical Pharmacology
12.1 Mechanism of Action
Leuprolide, a GnRH agonist, acts as an inhibitor of gonadotropin secretion. Animal and human studies indicate that following an initial stimulation of gonadotropins, chronic administration of leuprolide results in suppression of ovarian and testicular steroidogenesis. This effect is reversible upon discontinuation of drug therapy.
12.2 Pharmacodynamics
Mean serum testosterone concentrations transiently increased, then fell to below castrate threshold levels (≤ 50 ng/dL) within 3 weeks following administration of the dose of leuprolide, and generally remained below castrate thresholds levels throughout treatment.
In humans, subcutaneous administration of single daily doses of leuprolide result in an initial increase in circulating levels of LH and FSH, leading to a transient increase in levels of the gonadal steroids (testosterone and dihydrotestosterone in males). However, continuous daily administration of leuprolide results in decreased levels of LH and FSH. In males, testosterone is reduced to below castration levels. These decreases generally occur within 2 to 4 weeks after initiation of treatment, and castration levels of testosterone in prostatic cancer patients have been demonstrated for periods of up to 5 years.
12.3 Pharmacokinetics
Leuprolide concentration is variable, exhibiting an initial rapid increase followed by a rapid decline over the first 3 days before reaching steady concentrations for the duration of the dosing interval. The mean serum leuprolide C maxwas 94.5 and 99 ng/mL following the first and second doses of CAMCEVI, respectively. The mean serum concentration was maintained at 0.497–2.57 and 0.507–2.39 ng/ml after Day 3 post the first and second doses, respectively. The mean AUC 0-6 monwas 224 and 268 day.ng/mL following the first and second doses of CAMCEVI, respectively.
Absorption
The median T maxof leuprolide was 3.2 and 2.1 hours following the first and second doses of CAMCEVI, respectively
Distribution
The mean steady-state volume of distribution of leuprolide was 27 L following an intravenous bolus in healthy male volunteers. Protein binding of leuprolide ranged from 43% to 49% in vitro.
Elimination
The mean systemic clearance was 7.6 L/h and terminal elimination half-life of approximately 3 hours following an intravenous bolus of leuprolide in healthy male volunteers.
Metabolism
Administration of radiolabeled leuprolide was metabolized to smaller inactive peptides which may then be further catabolized.
Excretion
Excretion of leuprolide has not been evaluated with CAMCEVI.
Specific Populations
No clinically significant differences in the systemic exposure of leuprolide were observed based on age (51 to 88 years), race/ethnicity (White, Black, Asian), or body weight (54 to 134 kg). The effect of renal or hepatic impairment on the pharmacokinetics of leuprolide has not been evaluated.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Two-year carcinogenicity studies were conducted with leuprolide in rats and mice. In rats, a dose-related increase of benign pituitary hyperplasia and benign pituitary adenomas was noted at 24 months when the drug was administered subcutaneously at high daily doses (0.6 to 4 mg/kg). There was a significant but not dose-related increase of pancreatic islet-cell adenomas in females and of testicular interstitial cell adenomas in males (highest incidence in the low dose group). In mice, no pituitary abnormalities were observed at a dose as high as 60 mg/kg for 2 years. Patients have been treated with leuprolide for up to 3 years with doses as high as 10 mg/day and for 2 years with doses as high as 20 mg/day without demonstrable pituitary abnormalities.
Mutagenicity studies have been performed with leuprolide using bacterial and mammalian systems. These studies provided no evidence of mutagenic potential.
Leuprolide may reduce male and female fertility. Administration of leuprolide to male and female rats at doses of 0.024, 0.24, and 2.4 mg/kg as monthly depot formulation for up to 3 months (approximately as low as 1/30 of the human dose based on body surface area using an estimated daily dose in animals and humans) caused atrophy of the reproductive organs, and suppression of reproductive function. These changes were reversible upon cessation of treatment.
14. Clinical Studies
The efficacy of CAMCEVI was evaluated in an open label, single arm, multinational study FP01C-13-001 ( NCT02234115) in patients with advanced prostate carcinoma who have a baseline morning serum testosterone level >150 ng/dL and Eastern Cooperative Oncology Group performance status ≤ 2. CAMCEVI was administered subcutaneously at a dose of 42 mg initially on Day 0 and on Week 24.
The population (n = 137) had a median age of 71 years (range 51 to 88) and was 90% White, 6% Black, and 4% Asian. Disease stage was distributed as follows: 23% metastatic (M1), 27% locally advanced (T3/4 NX M0 or any T N1 M0), 26% localized (T1 or T2 N0 M0), and 24% not classifiable. The median testosterone concentration at baseline was 440 ng/dL.
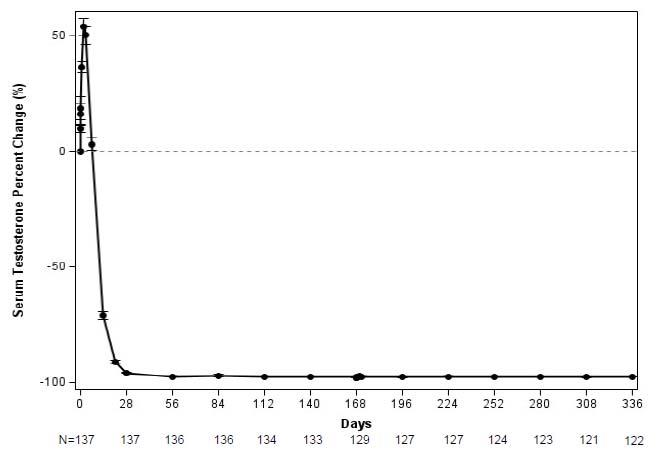
The major efficacy outcome measure was medical castration rate, defined as achieving and maintaining serum testosterone suppression to ≤ 50 ng/dL by Week 4 through Week 48 of treatment. Following the first injection of CAMCEVI, serum testosterone levels were suppressed to ≤ 50 ng/dL by Week 4 (+/-7 days) in 98.5% of the patients; and from Week 4 through Week 48 in 97.0% of patients (95% CI: 92.2-98.9) estimated using the Kaplan-Meier method. The time course of percent change from baseline in testosterone suppression are shown in Figure 1. The percentage of patients with testosterone suppression to ≤ 20 ng/dL was 69.3% on Day 28.
Figure 1 CAMCEVI Mean (95% CI) Percentage Change from Baseline in Serum Testosterone Concentration Over Time (N =137)
In the clinical trial, PSA levels were monitored and were lowered on average by 51% after 4 weeks after administration of CAMCEVI, 83% after 3 months and remained suppressed throughout the 48 weeks of treatment. These PSA results should be interpreted with caution because of the heterogeneity of the patient population studied. No evidence has shown that the rapidity of PSA decline correlates with clinical benefit.
16. How is Camcevi supplied
CAMCEVI is a sterile, off-white to pale yellow, viscous and opalescent injectable emulsion supplied in a kit as a single-dose, pre-filled syringe. CAMCEVI is available as follows:
|
Kit Contents |
NDC |
|
Injectable emulsion in a pre-filled syringe containing 42 mg leuprolide for subcutaneous injection, a sterile 18-gauge SurGuard ®3 safety needle, and Prescribing Information. |
69448-023-63 |
Store CAMCEVI at 2°C–8°C (36°F–46°F). Protect CAMCEVI from light by storing in the original package until time of use. Do not freeze or shake.
The rubber used in syringe tip cap and plunger stopper is not made of natural rubber latex.
17. Patient Counseling Information
Hypersensitivity
- Inform patients that if they have experienced hypersensitivity with other GnRH agonist drugs like CAMCEVI, CAMCEVI is contraindicated [see Contraindications ( 4)].
Tumor Flare
- Inform patients that CAMCEVI can cause tumor flare during the first weeks of treatment. Inform patients that the increase in testosterone can cause an increase in urinary symptoms or pain. Advise patients to contact their healthcare provider if uretral obstruction, spinal cord compression, paralysis, or new or worsened symptoms occur after beginning CAMCEVI treatment [see Warnings and Precautions ( 5.1)] .
Hyperglycemia and Diabetes
- Advise patients that there is an increased risk of hyperglycemia and diabetes with CAMCEVI therapy. Inform patients that periodic monitoring for hyperglycemia and diabetes is required when being treated with CAMCEVI [see Warnings and Precautions ( 5.2)] .
Cardiovascular Diseases
- Inform patients that there is an increased risk of myocardial infarction, sudden cardiac death, and stroke with CAMCEVI treatment. Advise patients to immediately report signs and symptoms associated with these events to their healthcare provider for evaluation [see Warnings and Precautions ( 5.3)].
QT/QTc Prolongation
- Inform patients that CAMCEVI can cause QT/QTc prolongation. Advise patients to immediately contact their healthcare provider in the event of syncope, presyncopal symptoms, or cardiac palpitations [see Warnings and Precautions ( 5.4)].
Convulsions
- Inform patients that there is an increased risk of convulsions with CAMCEVI treatment. Advise patients to immediately contact their healthcare provider if they experience convulsions [see Warnings and Precautions ( 5.5)].
Injection Site Reactions
- Inform patients that injection site related adverse reactions may occur such as transient burning/stinging, pain, bruising, and redness. Advise patients to contact their healthcare provider if they experience rash or severe injection site reactions [see Adverse Reactions ( 6.1)].
Urogenital Disorders
- Advise patients that CAMCEVI may cause impotence.
Infertility
- Inform patients that CAMCEVI may cause infertility [see Use In Specific Populations ( 8.3)].
Manufactured for:
Accord BioPharma Inc.
8041 Arco Corporate Drive, Suite 200
Raleigh, NC 27617, USA
By:
Fareva Pau
Fareva Pau 1, Avenue du Bearn, IDRON, 64320, France
| CAMCEVI
leuprolide injection, emulsion |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Accord BioPharma, Inc. (079636487) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Fareva Pau | 273960691 | manufacture(69448-023) , analysis(69448-023) | |
Frequently asked questions
- Will I get my period while on Lupron?
- Are Lupron Depot and Eligard the same drug?
- What does Lupron do for IVF?
- Can you get pregnant on Lupron Depot?
- Is Firmagon (degarelix) the same as Lupron Depot?
More about Camcevi (leuprolide)
- Check interactions
- Compare alternatives
- Drug images
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- Drug class: gonadotropin releasing hormones
Patient resources
Professional resources
Other brands
Eligard, Lupron Depot, Lupron Depot-PED, Lupron Depot-Gyn, ... +2 more