Tabradol: Package Insert / Prescribing Info
Package insert / product label
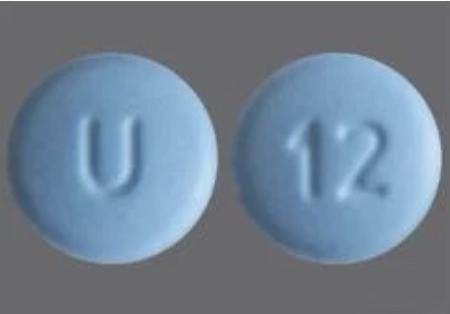
Generic name: cyclobenzaprine hydrochloride
Dosage form: kit
Drug class: Skeletal muscle relaxants
Medically reviewed by Drugs.com. Last updated on Mar 3, 2025.
Tabradol Description

Tabradol Description

Tabradol Description

Instructions

Related/similar drugs
Instructions

Tabradol Description

Principal Display Panel

TABRADOL
cyclobenzaprine hydrochloride kit |
|
|
|
|
|
|
| Part 1 of 3 |
CYCLOBENZAPRINE HYDROCHLORIDE
cyclobenzaprine hydrochloride powder, for suspension |
|
|
|
|
|
|
|
|
|
|
|
| Part 2 of 3 |
STRUCTURED SUSPENSION VEHICLE
suspension liquid |
|
|
|
|
|
|
|
|
|
|
|
| Part 3 of 3 |
STRUCTURED FLAVORING VEHICLE
flavor liquid |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Frequently asked questions
View more FAQ
More about cyclobenzaprine
Patient resources
Professional resources
Other brands
Flexeril, Tonmya, Amrix, Fexmid
Related treatment guides
Medical Disclaimer