Unichem Pharmaceuticals (USA) Inc. Issues Voluntary Nationwide Recall of Cyclobenzaprine Hydrochloride Tablets USP 10 mg, Due to Mislabeling
Audience: Consumer, Pharmacy, Health Care Professional
August 27, 2025– East Brunswick, NJ, Unichem Pharmaceuticals (USA), Inc. is voluntarily recalling one (1) lot of Cyclobenzaprine Hydrochloride Tablets USP 10 mg, to the consumer level. The Cyclobenzaprine 10mg (90ct) label was inadvertently placed on a bottle containing Meloxicam 7.5 mg tablets.
Risk Statement: For patients who unknowingly take Meloxicam there is a reasonable probability of serious adverse events including cardiovascular, gastrointestinal, renal, anaphylaxis, and skin reactions, particularly in those patients taking concomitant non-steroidal anti-Inflammatory drugs and/or blood thinners, those who have allergies to the Meloxicam, or those with underlying illness. To date, Unichem Pharmaceuticals has not received any reports of adverse events related to this recall.
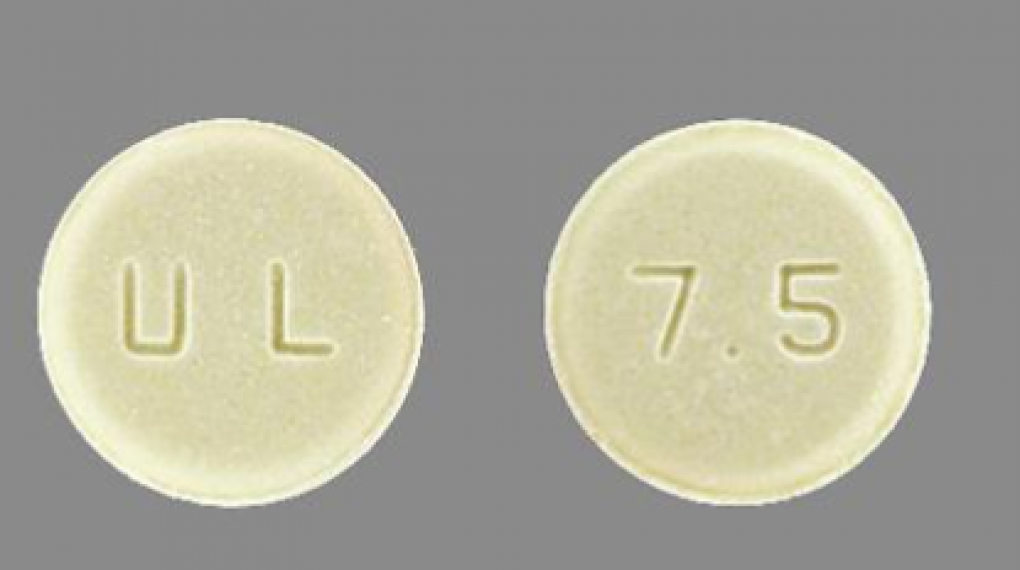
Meloxicam Tablets USP, 7.5 mg is a non-steroidal anti-inflammatory drug, indicated for use in Osteoarthritis, Rheumatoid Arthritis, and Juvenile Rheumatoid Arthritis. Meloxicam Tablets, USP, 7.5 mg is light yellow, round flat beveled edged, tablet with “U & L” debossed on one side and “7.5” debossed centrally on the other side.
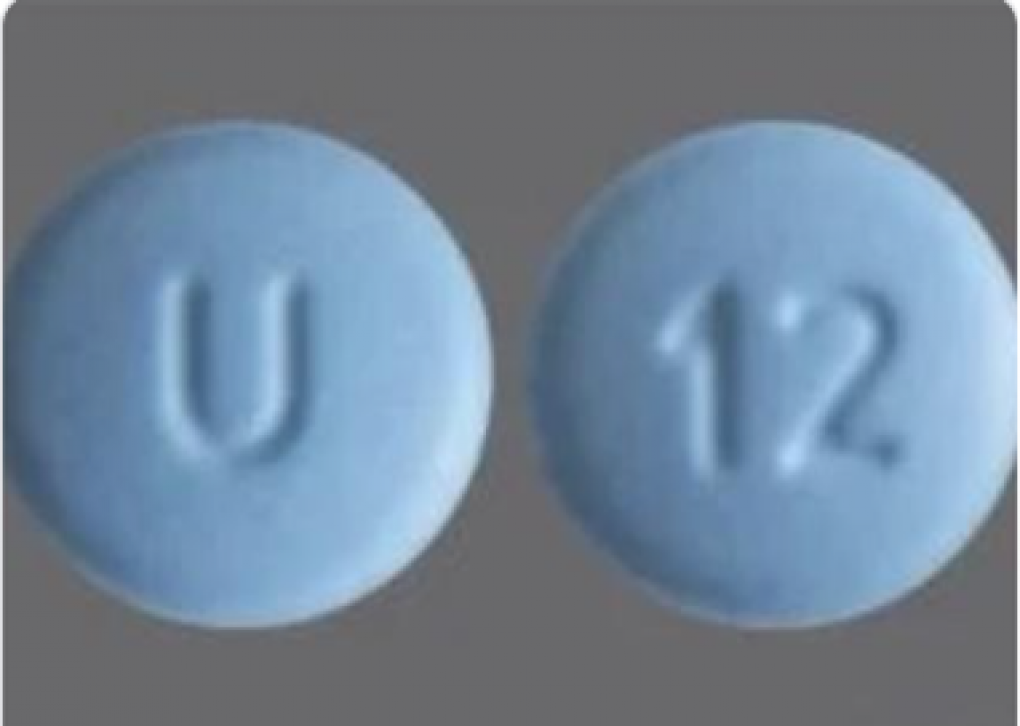
Cyclobenzaprine Hydrochloride Tablets USP, 10mg is a muscle relaxer and indicated as an adjunct to rest and physical therapy for relief of muscle spasm associated with acute, painful musculoskeletal conditions. Cyclobenzaprine Hydrochloride Tablets, USP, 10 mg, are blue colored, film coated, round shaped, biconvex tablets, debossed with “U” on one side and “12” debossed on other side.
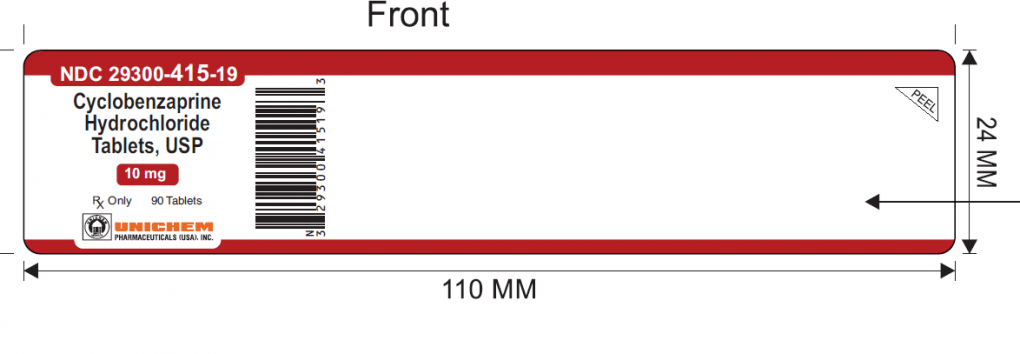
The mislabeled bottles of Cyclobenzaprine Hydrochloride Tablets USP, 10mg but containing Meloxicam 7.5mg tablets, can be identified by the lot number GMML24026A and expiry of Sept 2027 and NDC 29300-415-19 printed on the label of the 90-count bottles.
The product was distributed Nationwide to distributors, and further downstream distribution occurred to retailers and subsequently consumers.
Unichem Pharmaceuticals (USA), Inc. is notifying its downstream trading partners, their retailers and consumers of the recall through our third party recall provider, Inmar. Inmar is arranging for the return of the subject recalled Cyclobenzaprine Hydrochloride Tablets USP, 10mg labeled with Lot # GMML24026A. Our downstream trading partners that have Cyclobenzaprine Hydrochloride Tablets USP, 10mg with Lot# GMML24026A, Exp Sept 2027, which is being recalled, should not further distribute, this medication, and notify their customers accordingly. Retail pharmacies should not dispense from this lot number, GMML24026A, and call the number provided for guidance on how to return this drug product. Any pharmacy who has dispensed this lot of Cyclobenzaprine, should notify the consumer. Consumers should return the medication to the pharmacy they received their prescription from.
Consumers with questions regarding this recall can Inmar at 1-877-840-5109 or via email a to rxrecalls@inmar.com; Monday – Friday (9 am – 5 pm; CST). Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA’s MedWatch Adverse Event Reporting program either online, by regular mail, or by fax.
- Complete and submit the report online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form or submit by fax to 1-800-FDA-0178.
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
The Product Label Subject to this recall is shown below:

More news resources
- FDA Medwatch Drug Alerts
- Daily MedNews
- News for Health Professionals
- New Drug Approvals
- New Drug Applications
- Drug Shortages
- Clinical Trial Results
- Generic Drug Approvals
Subscribe to our newsletter
Whatever your topic of interest, subscribe to our newsletters to get the best of Drugs.com in your inbox.

