707 SP 7.5 Pill: pink, round, 6mm
Generic Name: moexipril
The pill with imprint 707 SP 7.5 (Pink, Round, 6mm) has been identified as Univasc 7.5 mg and is used for Diabetic Kidney Disease, Heart Attack, Heart Failure, High Blood Pressure, and Left Ventricular Dysfunction. It belongs to the drug class Angiotensin Converting Enzyme Inhibitors and is not a controlled substance.
Images for 707 SP 7.5
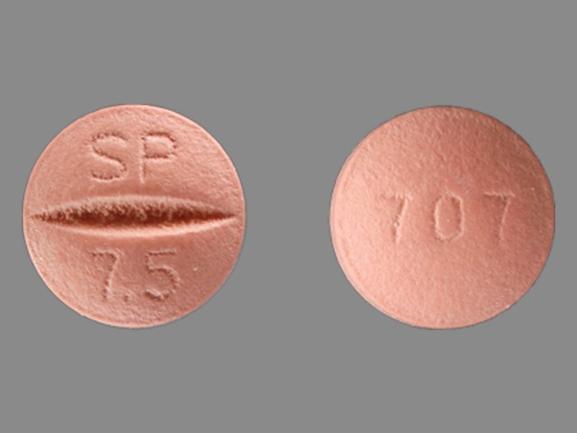



Univasc
- Generic Name
- moexipril
- Imprint
- 707 SP 7.5
- Strength
- 7.5 mg
- Color
- Pink
- Size
- 6.00 mm
- Shape
- Round
- Availability
- Prescription only
- Drug Class
- Angiotensin Converting Enzyme Inhibitors
- Pregnancy Category
- D - Positive evidence of risk
- CSA Schedule
- Not a controlled drug
- Labeler / Supplier
- Schwarz Pharma
- National Drug Code (NDC)
- 00091-3707 (Discontinued)
- Inactive Ingredients
-
lactose,
magnesium oxide,
crospovidone,
magnesium stearate,
gelatin,
hydroxypropyl cellulose,
hypromelloses,
polyethylene glycol 6000,
titanium dioxide,
ferric oxide red
Note: Inactive ingredients may vary.
See also:
More about Univasc (moexipril)
- Check interactions
- Compare alternatives
- Reviews (2)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Drug class: Angiotensin Converting Enzyme Inhibitors
- Breastfeeding
Patient resources
Professional resources
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
