Varubi: Package Insert / Prescribing Info
Package insert / product label
Generic name: rolapitant
Dosage form: tablet
Drug class: NK1 receptor antagonists
J Code (medical billing code): J8670 (1 mg, oral)
Medically reviewed by Drugs.com. Last updated on Sep 6, 2024.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Drug Interactions
- Use In Specific Populations
- Overdosage
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
VARUBI® (rolapitant) tablets, for oral use
Initial U.S. Approval: 2015
Indications and Usage for Varubi
VARUBI is a substance P/neurokinin 1 (NK1) receptor antagonist indicated in combination with other antiemetic agents in adults for the prevention of delayed nausea and vomiting associated with initial and repeat courses of emetogenic cancer chemotherapy, including, but not limited to, highly emetogenic chemotherapy. (1)
Varubi Dosage and Administration
- Administer in combination with dexamethasone and a 5-HT3 receptor antagonist; see full prescribing information for dosing information. (2)
- No dosage adjustment for dexamethasone is required. (2)
- The recommended dosage of VARUBI is 180 mg as a single dose orally within 2 hours prior to the initiation of chemotherapy on Day 1. (2)
- Administer VARUBI prior to the initiation of each chemotherapy cycle, but at no less than 2 week intervals. (2)
- Administer VARUBI without regards to meals. (2)
Dosage Forms and Strengths
Tablets: 90 mg of rolapitant (3)
Contraindications
Warnings and Precautions
CYP2D6 Substrates: Rolapitant is a moderate inhibitor of CYP2D6 and significantly increases the plasma concentrations of CYP2D6 substrates for at least 28 days following single dose administration of VARUBI. Before starting VARUBI, consider if patients require:
- thioridazine or pimozide; if so, use an alternative antiemetic to VARUBI or an alternative to thioridazine or pimozide that is not metabolized by CYP2D6.
- other CYP2D6 substrates; if so, consult the prescribing information for the CYP2D6 substrate for additional information about interactions with CYP2D6 inhibitors. (4, 5.1, 7)
Adverse Reactions/Side Effects
Most common adverse reactions (≥3%) are:
- Cisplatin Based Highly Emetogenic Chemotherapy: neutropenia, hiccups and abdominal pain. (6.1)
-
Moderately Emetogenic Chemotherapy and Combinations of
Anthracycline and Cyclophosphamide: decreased appetite, neutropenia, dizziness, dyspepsia, urinary tract infection, stomatitis and anemia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact TerSera Therapeutics at 1-844-334-4035 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Strong CYP3A4 Inducers (e.g., rifampin): significantly reduced plasma concentrations of rolapitant can decrease the efficacy of VARUBI; avoid use of VARUBI in patients who require chronic administration of such drugs. (7)
- BCRP and P-gp Substrates with a Narrow Therapeutic Index: Oral VARUBI is an inhibitor of BCRP and P-gp and can increase plasma concentrations of the concomitant drug and potential for adverse reactions. See full prescribing information for specific examples. (7)
- Warfarin: Monitor for increased INR or prothrombin time; adjust the dose of warfarin as needed. (7)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 8/2020
Full Prescribing Information
1. Indications and Usage for Varubi
VARUBI® is indicated in combination with other antiemetic agents in adults for the prevention of delayed nausea and vomiting associated with initial and repeat courses of emetogenic cancer chemotherapy, including, but not limited to, highly emetogenic chemotherapy.
2. Varubi Dosage and Administration
The recommended dosage of VARUBI in adults in combination with a 5-HT3 receptor antagonist and dexamethasone for the prevention of nausea and vomiting with emetogenic cancer chemotherapy is shown in Table 1. There is no drug interaction between rolapitant and dexamethasone, so no dosage adjustment for dexamethasone is required. Administer a dexamethasone dose of 20 mg on Day 1 [see Clinical Pharmacology (12.3)].
Administer VARUBI prior to the initiation of each chemotherapy cycle, but at no less than 2 week intervals.
Administer VARUBI without regards to meals.
| Day 1 | Day 2 | Day 3 | Day 4 | |
| Prevention of Nausea and Vomiting Associated with Cisplatin-Based Highly Emetogenic Cancer Chemotherapy | ||||
| VARUBI | 180 mg as a single dose orally within 2 hours prior to initiation of chemotherapy | None | ||
| Dexamethasone | 20 mg; 30 min prior to initiation of chemotherapy | 8 mg twice daily | 8 mg twice daily | 8 mg twice daily |
| 5-HT3 receptor antagonist | See the prescribing information for the co-administered 5-HT3 receptor antagonist for appropriate dosing information. | None | ||
| Prevention of Nausea and Vomiting Associated with Moderately Emetogenic Cancer Chemotherapy and Combinations of Anthracycline and Cyclophosphamide | ||||
| VARUBI | 180 mg as a single dose orally within 2 hours prior to initiation of chemotherapy | None | ||
| Dexamethasone | 20 mg; 30 min prior to initiation of chemotherapy | None | ||
| 5-HT3 receptor antagonist | See the prescribing information for the co-administered 5-HT3 receptor antagonist for appropriate dosing information. | See the prescribing information for the co-administered 5-HT3 receptor antagonist for appropriate dosing information. | ||
3. Dosage Forms and Strengths
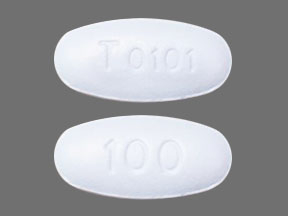
Tablets: 90 mg rolapitant; film-coated, blue capsule shaped, debossed with T0101 on one side and 100 on the other side.
4. Contraindications
VARUBI is contraindicated in patients taking CYP2D6 substrates with a narrow therapeutic index, such as thioridazine and pimozide. VARUBI can significantly increase the plasma concentrations of thioridazine and pimozide, which may result in QT prolongation and Torsades de Pointes [see Warnings and Precautions (5.1)].
VARUBI is contraindicated in pediatric patients less than 2 years of age because of irreversible impairment of sexual development and fertility observed in juvenile rats at clinically relevant dosages [see Use in Specific Populations (8.4)].
5. Warnings and Precautions
5.1 Interaction with CYP2D6 Substrates
Rolapitant is a moderate inhibitor of CYP2D6. Exposure to dextromethorphan, a CYP2D6 substrate, following a single dose of rolapitant increased about 3-fold on Days 8 and Day 22. The inhibition of CYP2D6 persisted on Day 28 with a 2.3-fold increase in dextromethorphan (CYP2D6 substrate) concentrations, the last time point measured. The inhibitory effect of rolapitant on CYP2D6 is expected to persist beyond 28 days for an unknown duration following administration of VARUBI [see Drug Interactions (7), Clinical Pharmacology (12.3)].
Narrow Therapeutic Index Drugs (Thioridazine and Pimozide)
VARUBI is contraindicated in patients taking CYP2D6 substrates with a narrow therapeutic index such as thioridazine and pimozide. Increased plasma concentrations of thioridazine and pimozide are associated with serious and/or life-threatening events of QT prolongation and Torsades de Pointes [see Contraindications (4)].
Before starting treatment with VARUBI, consider whether patients require treatment with thioridazine or pimozide. If patients require these drugs, use an alternative antiemetic to VARUBI or an alternative to thioridazine or pimozide that is not metabolized by CYP2D6.
Other Drugs
VARUBI can also increase plasma concentrations of other CYP2D6 substrates for at least 28 days following administration of VARUBI and may result in adverse reactions.
Before starting treatment with VARUBI, consult the prescribing information for CYP2D6 substrates to obtain additional information about interactions with CYP2D6 inhibitors.
6. Adverse Reactions/Side Effects
The following adverse reactions are discussed in greater detail in other sections of the labeling:
- Interaction with CYP2D6 Substrates [see Contraindications (4), Warnings and Precautions (5.1)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
In 4 controlled clinical trials in patients receiving emetogenic cancer chemotherapy, VARUBI was given in combination with a 5-HT3 receptor antagonist and dexamethasone. On Day 1 of Cycle 1 of chemotherapy, 1567 patients were treated with VARUBI and 1198 of these patients continued into the optional multiple cycle extension for up to 6 cycles of chemotherapy. The median number of cycles administered 180 mg of VARUBI was four. VARUBI 180 mg was administered to 1294 patients.
In Cycle 1 adverse reactions were reported in approximately 7% of patients treated with VARUBI compared with approximately 6% of patients treated with control therapy. The most common adverse reactions reported with an incidence of ≥3% and greater than control are listed in Table 2 and Table 3.
|
* all reactions occurring at ≥3% in the VARUBI group and for which the rate for VARUBI exceeds the rate for control |
||
| VARUBI Regimen
(VARUBI, Dexamethasone, and 5-HT3 Receptor Antagonist) N = 624 | Control
(Placebo, Dexamethasone, and 5-HT3 Receptor Antagonist) N = 627 |
|
| Neutropenia | 9% | 8% |
| Hiccups | 5% | 4% |
| Abdominal Pain | 3% | 2% |
|
*all reactions occurring at ≥3% in the VARUBI group and for which the rate for VARUBI exceeds the rate for control. |
||
| VARUBI Regimen
(VARUBI, Dexamethasone, and 5-HT3 Receptor Antagonist) N = 670 | Control
(Placebo, Dexamethasone, and 5-HT3 Receptor Antagonist) N = 674 |
|
| Decreased appetite | 9% | 7% |
| Neutropenia | 7% | 6% |
| Dizziness | 6% | 4% |
| Dyspepsia | 4% | 2% |
| Urinary tract infection | 4% | 3% |
| Stomatitis | 4% | 2% |
| Anemia | 3% | 2% |
Adverse reactions in the multiple-cycle extensions of highly and moderately emetogenic chemotherapy studies for up to 6 cycles of chemotherapy were generally similar to that observed in Cycle 1.
Related/similar drugs
7. Drug Interactions
Rolapitant is a moderate CYP2D6 inhibitor. The inhibition of CYP2D6 persisted on Day 28 with a 2.3-fold increase in dextromethorphan concentrations, the last time point measured. The inhibitory effect of rolapitant on CYP2D6 is expected to persist beyond 28 days for an unknown duration following administration of VARUBI [see Clinical Pharmacology (12.3)].
Oral rolapitant is an inhibitor of Breast-Cancer-Resistance Protein (BCRP) and p-glycoprotein (P-gp). Rolapitant, given as a single oral dose, is not an inhibitor or inducer of CYP3A4 [see Clinical Pharmacology (12.3)]. Therefore, no dosage adjustment for dexamethasone (CYP3A4 substrate) is needed when co-administered with VARUBI [see Dosage and Administration (2)].
Table 4 and Table 5 include drugs with clinically important drug interactions when administered concomitantly with VARUBI and instructions for preventing or managing them.
| CYP2D6 Substrates | |
| Narrow Therapeutic Index Drugs (Thioridazine and Pimozide) | |
| Clinical Impact: | Increased plasma concentrations of thioridazine and pimozide are associated with serious and/or life-threatening events of QT prolongation and Torsades de Pointes. |
| Intervention: | VARUBI is contraindicated in patients taking CYP2D6 substrates with a narrow therapeutic index such as thioridazine and pimozide. If patients require these drugs, use an alternative antiemetic to VARUBI or an alternative to thioridazine or pimozide that is not metabolized by CYP2D6 [see Contraindications (4), Warnings and Precautions (5.1)]. |
| Other CYP2D6 Substrates | |
| Clinical Impact: | VARUBI can increase plasma concentrations of other CYP2D6 substrates for at least 28 days following administration of VARUBI and may result in adverse reactions. Before starting treatment with VARUBI consult the prescribing information of CYP2D6 substrates to obtain further information about interactions with CYP2D6 inhibitors [see Clinical Pharmacology (12.3)]. |
| Intervention: | Before starting treatment with VARUBI consult the prescribing information of CYP2D6 substrates to obtain further information about interactions with CYP2D6 inhibitors [see Warnings and Precautions (5.1)]. |
| BCRP Substrates with a Narrow Therapeutic Index (e.g., methotrexate, topotecan, or irinotecan) | |
| Clinical Impact: | Increased plasma concentrations of BCRP substrates (e.g., methotrexate, topotecan, or irinotecan) may result in potential adverse reactions [see Clinical Pharmacology (12.3)]. |
| Intervention: | Monitor for adverse reactions related to the concomitant drug if use of VARUBI cannot be avoided. Use the lowest effective dose of rosuvastatin (see prescribing information for additional information on recommended dosing). |
| P-gp Substrates with a Narrow Therapeutic Index (e.g. digoxin) | |
| Clinical Impact: | Increased plasma concentrations of P-gp substrates (e.g., digoxin) may result in potential adverse reactions [see Clinical Pharmacology (12.3)]. |
| Intervention: | Monitor digoxin concentrations with concomitant use of VARUBI and adjust the dosage as needed to maintain therapeutic concentrations. Monitor for adverse reactions if concomitant use of VARUBI with other P-gp substrates with a narrow therapeutic index cannot be avoided. |
| Warfarin | |
| Clinical Impact: | Although co-administration of intravenous rolapitant (VARBI is not approved for intravenous use), which has a higher Cmax than oral VARUBI, with warfarin did not substantially increase the systemic exposure to S-warfarin, the active enantiomer, the effects on INR and prothrombin time were not studied [see Clinical Pharmacology (12.3)]. |
| Intervention: | Monitor INR and prothrombin time and adjust the dosage of warfarin, as needed with concomitant use of VARUBI, to maintain the target INR range. |
| Strong CYP3A4 Inducers (e.g. rifampin) | |
| Clinical Impact: | Co-administration of VARUBI with rifampin can significantly reduce the plasma concentrations of rolapitant and decrease the efficacy of VARUBI [see Clinical Pharmacology (12.3)]. |
| Intervention: | Avoid the use of VARUBI in patients who require chronic administration of strong CYP3A4 inducers. |
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
The limited data with VARUBI use in pregnant women are insufficient to inform a drug associated risk of adverse developmental outcomes. In animal reproduction studies, there were no adverse developmental effects observed with oral administration of rolapitant in rats and rabbits during the period of organogenesis at doses up to 1.2 times and 2.9-times, respectively, the maximum recommended human dose (MRHD) (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
The potential embryo-fetal toxicity of rolapitant was assessed in pregnant rats administered oral doses up to 22.5 mg/kg per day throughout the period of organogenesis. Rats administered doses of 13.5 or 22.5 mg/kg per day rolapitant exhibited evidence of maternal toxicity including decreased body weight gain and/or body weight loss and a concomitant decrease in food consumption during the first week of dosing. No adverse embryo-fetal developmental effects were observed at doses up to 22.5 mg/kg per day rolapitant (approximately 1.2 times the recommended human dose on a body surface area basis). In rabbits administered rolapitant throughout the period of organogenesis, oral doses up to 27 mg/kg per day (approximately 2.9 times the recommended human dose on a body surface area basis) were without effects on the developing fetus.
The pre- and postnatal developmental effects of rolapitant were assessed in rats administered oral doses of 2.25, 9 or 22.5 mg/kg per day during the periods of organogenesis and lactation. Maternal toxicity was evident based on mortality/moribund condition, decreased body weight and food consumption, total litter loss, prolonged parturition, decreased length of gestation, and increased number of unaccounted for implantation sites at a dose of 22.5 mg/kg per day (approximately 1.2 times the recommended human dose on a body surface area basis). Effects on offspring at this dose included decreased postnatal survival, and decreased body weights and body weight gain, and may be related to the maternal toxicity observed. At a maternal dose of 9 mg/kg per day rolapitant (approximately 0.5 times the recommended human dose on a body surface area basis), there was a decrease in memory in female pups in a maze test and a decrease in pup body weight.
8.2 Lactation
Risk Summary
There are no data on the presence of rolapitant in human milk, the effects of rolapitant in the breastfed infant, or the effects of rolapitant on milk production. Rolapitant administered orally to lactating female rats was present in milk (see Data). The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for VARUBI and any potential adverse effects on the breastfed infant from VARUBI or from the underlying maternal condition or the use of concomitant chemotherapy.
Data
Radioactivity from labeled [14C] rolapitant was transferred into milk of lactating rats following a single oral dose of 22.5 mg/kg, and the maximum radioactivity in milk was observed at 12 hours post-dose. The mean milk/plasma radioactivity concentration ratios in dams at 1 to 48 hours post-dose ranged from 1.24 to 3.25. Based on average daily consumption of milk (2 mL/day) and the maximum milk radioactivity determined, pup exposure is expected to be 0.3% of the orally administered dose.
8.3 Females and Males of Reproductive Potential
Infertility
Females
In animal fertility studies, rolapitant impaired the fertility in females in a reversible fashion [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
The safety and effectiveness of VARUBI have not been established in pediatric patients. VARUBI is contraindicated in pediatric patients less than 2 years of age [see Contraindications(4)]. Rolapitant administration in juvenile rats (human age equivalent of birth to 2 years) resulted in abnormal ovarian and uterine development, early sexual development in females, delayed sexual development in males, and impaired fertility.
Juvenile Animal Toxicity Data
A toxicity study in juvenile rats at rolapitant doses approximately 1.2 and 2.5 times the approved adult body surface area (BSA)-based dose from postnatal day (PND) 7 through PND 70 (approximate human age equivalent of birth to 16 years) identified reproductive toxicity. A subsequent toxicity study in juvenile rats was conducted to identify the critical window of exposure for reproductive toxicity. A rolapitant dose of 50 mg/kg/day (approximately 2.7 times the approved adult BSA-based dose) was administered daily from PNDs 7 through 70, 7 to 21, 21 to 42 and 42 to 70 (approximate human age equivalent of birth to 16 years, birth to 2 years, 2 years to 12 years, and 12 years to 16 years, respectively). Female juvenile rats treated with rolapitant beginning on PND 7 developed adverse effects including partial or irreversible lower uterine weights that correlated with decreased endometrial glands of the uterus, decreases in the numbers of corpora lutea, implantation sites and live embryos and increases in pre- and post-implantation loss, and early resorptions. These adverse effects were observed in female juvenile rats administered rolapitant prior to PND 21 (approximate human age equivalent of 2 years). Additionally, juvenile rats treated with rolapitant beginning on either PND 7 or PND 21 developed slight changes in the onset of sexual maturation (including earlier attainment of vaginal opening in females and delay in preputial separation in males).
8.5 Geriatric Use
Of the 1294 subjects treated with VARUBI, 25% were 65 years and over, while 5% were 75 and over. No overall differences in safety or efficacy were reported between the elderly subjects and younger subjects, but greater sensitivity of some older individuals cannot be ruled out.
8.6 Hepatic Impairment
No dosage adjustment is needed in patients with mild (Child-Pugh Class A) or moderate (Child-Pugh Class B) hepatic impairment. There are no clinical or pharmacokinetic data in patients with severe hepatic impairment (Child-Pugh Class C). Avoid use of VARUBI in patients with severe hepatic impairment. If use cannot be avoided, monitor patients for adverse reactions related to rolapitant [see Adverse Reactions (6.1)].
10. Overdosage
There are no data on overdose with VARUBI.
There is no antidote for VARUBI overdose. Discontinue VARUBI in the event of overdose, and institute general supportive measures and close observation.
11. Varubi Description
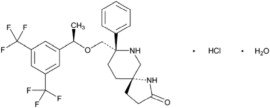
VARUBI contains rolapitant, a substance P/neurokinin 1 (NK1) receptor antagonist. Rolapitant hydrochloride is chemically described as (5S,8S)-8- { [(1R)-1-[3,5-bis(trifluoromethyl)phenyl]ethoxy]methyl]}-8-phenyl-1,7-diazaspiro[4.5]decan-2-one hydrochloride. Its empirical formula is C25H26F6N2O2. HCl.H2O, and its structural formula is:
Rolapitant hydrochloride is a white to off-white powder, with a molecular weight of 554.95. Solubility of rolapitant hydrochloride in aqueous solution is pH-dependent and is more soluble at lower pH. Rolapitant has good solubility in common pharmaceutical solvents such as ethanol, propylene glycol and 40% hydroxypropyl beta-cyclodextrin.
Each tablet contains 90 mg rolapitant (equivalent to 100 mg rolapitant hydrochloride) and the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, povidone, and pregelatinized starch. The tablets are coated in non-functional blue and clear coats. The tablet coating comprises the following inactive ingredients: FD&C Blue No. 2-Indigo Carmine Lake, polyethylene glycol, polysorbate 80, polyvinyl alcohol, talc, and titanium dioxide.
12. Varubi - Clinical Pharmacology
12.1 Mechanism of Action
Rolapitant is a selective and competitive antagonist of human substance P/NK1 receptors. Rolapitant does not have significant affinity for the NK2 or NK3 receptors or for a battery of other receptors, transporters, enzymes and ion channels. Rolapitant is also active in animal models of chemotherapy-induced emesis.
12.2 Pharmacodynamics
NK1 Receptor Occupancy
A human Positron Emission Tomography (PET) study with rolapitant demonstrated that rolapitant crosses the blood brain barrier and occupies brain NK1 receptors. A dose-dependent increase in mean NK1 receptor occupancy was observed in the oral dose range from 4.5 mg to 180 mg of rolapitant. At the 180 mg oral dose of rolapitant, the mean NK1 receptor occupancy was 73% in the striatum at 120 hours after a single dose administration in healthy subjects. The relationship between NK1 receptor occupancy and the clinical efficacy of rolapitant has not been established.
12.3 Pharmacokinetics
Absorption
Following a single oral dose administration of 180 mg VARUBI under fasting conditions to healthy subjects, rolapitant was measurable in plasma within 30 minutes and the peak plasma concentration (Cmax) for rolapitant which was reached in about 4 hours and mean Cmax was 968 ng/mL (%CV:28%).
Following multiple oral doses of 9 to 45 mg once daily of rolapitant (5% to 25% of the recommended dose) for 10 days, accumulation of rolapitant (ratio of AUC0-24hr) ranged from 5.0 to 5.3 fold.
The systemic exposures (Cmax and AUC) to rolapitant increased in a dose-proportional manner when single oral doses of rolapitant increased from 4.5 mg to 180 mg. With 4 times the recommended clinical oral dose of 180 mg, the Cmax and AUC of rolapitant increased 3.1 fold and 3.7 fold, respectively.
Concomitant administration of a high-fat meal did not significantly affect the pharmacokinetics of rolapitant after administration of 180 mg VARUBI [see Dosage and Administration (2)].
Distribution
Rolapitant was highly protein bound to human plasma (99.8%). The apparent volume of distribution (Vd/F) following a single oral dose of 180 mg rolapitant was 460 L in healthy subjects. The large Vd indicated an extensive tissue distribution of rolapitant. In a population pharmacokinetic analysis of oral rolapitant, the Vd/F was 387 L in cancer patients.
Elimination
Following single oral doses (4.5 to 180 mg) of rolapitant, the mean terminal half-life (t1/2) of rolapitant ranged from 169 to 183 hours (approximately 7 days), and was independent of dose. In a population pharmacokinetic analysis, the apparent total clearance (CL/F) of oral rolapitant was 0.96 L/hour in cancer patients.
Metabolism
Rolapitant is metabolized primarily by CYP3A4 to form a major active metabolite, M19 (C4-pyrrolidine-hydroxylated rolapitant). In a mass balance study, the metabolite M19 was determined to be the major circulating metabolite. The rate of formation of M19 was relatively slow, resulting in the delayed median Tmax of 120 hours (range: 24 to 168 hours) with Cmax of 183 ng/mL. The mean half-life of M19 was 158 hours.
The exposure ratio of M19 to rolapitant was approximately 50% in plasma.
Excretion
Rolapitant is eliminated primarily through the hepato/biliary route. Following administration of a single oral 180-mg dose of [14C]-rolapitant, on average 14.2% (range 9% to 20%) and 73% (range 52% to 89%) of the dose was recovered in the urine and feces, respectively over 6 weeks. In pooled samples collected over 2 weeks, 8.3% of the dose was recovered in the urine primarily as metabolites and 37.8% of the dose was recovered in the feces primarily as unchanged rolapitant. Unchanged rolapitant or M19 was not found in pooled urine sample.
Specific Populations
Age, Male and Female Patients and Racial or Ethnic Groups
Population pharmacokinetic analyses indicated that age, sex and race had no significant impact on the pharmacokinetics of rolapitant.
Patients with Hepatic Impairment
Following administration of a single oral dose of 180 mg rolapitant to patients with mild hepatic impairment (Child-Pugh Class A), the pharmacokinetics of rolapitant were comparable with those of healthy subjects. In patients with moderate hepatic impairment (Child-Pugh Class B), the mean Cmax was 25% lower while mean AUC of rolapitant was similar compared to those of healthy subjects. The median Tmax for M19 was delayed to 204 hours in patients with mild or moderate hepatic impairment compared to 168 hours in healthy subjects. The pharmacokinetics of rolapitant were not studied in patients with severe hepatic impairment (Child-Pugh Class C) [see Use in Specific Populations (8.6)].
Patients with Renal Impairment
In population pharmacokinetic analyses, rolapitant pharmacokinetics in cancer patients with mild (CLcr: 60 to 90 mL/min) or moderate (CLcr: 30 to 60 mL/min) renal impairment was comparable to cancer patients with normal kidney function. Information is insufficient for the effect of severe renal impairment. The pharmacokinetics of rolapitant was not studied in patients with end-stage renal disease requiring hemodialysis.
Drug Interaction Studies
Effect of Other Drugs on Rolapitant
Rolapitant is a substrate for CYP3A4.
-
CYP3A4 inducers
- When 600 mg rifampin was administered once daily for 7 days before and 7 days after administration of a single oral dose of 180 mg rolapitant, the mean Cmax of rolapitant was reduced by 30% and the mean AUC was reduced by 85% compared to administration of rolapitant alone. The mean half-life of rolapitant decreased from 176 hours without rifampin to 41 hours with concurrent rifampin [see Drug Interactions (7)].
-
CYP3A4 inhibitors
- Concurrent administration of 400 mg ketoconazole, a strong CYP3A4 inhibitor, once daily for 21 days following a single 90 mg oral dose of rolapitant, did not affect the Cmax of rolapitant while the AUC increased by 21%. These pharmacokinetic differences are not clinically significant.
Effect of Rolapitant on Other Drugs
The effect of VARUBI on CYP450 enzymes and transporters is summarized below.
-
CYP3A4 substrates
- Rolapitant is neither an inhibitor nor an inducer of CYP3A4.
- Midazolam: A single oral dose of 180 mg rolapitant had no significant effects on the pharmacokinetics of midazolam when oral midazolam 3 mg was co-administered on Day 1 and administered alone on Days 6 and 9.
- Ondansetron: Rolapitant had no significant effects on the pharmacokinetics of intravenous ondansetron when concomitantly administered with a single 180 mg oral dose of rolapitant on the same day.
- Dexamethasone: Rolapitant had no significant effects on the pharmacokinetics of dexamethasone when oral dexamethasone was administered on Days 1 to 3 after a single 180 mg oral dose of rolapitant was co-administered on Day 1 [see Dosage and Administration (2)].
-
CYP2D6 substrates
- Rolapitant is a moderate inhibitor of CYP2D6 [see Contraindications (4), Warnings and Precautions (5.1), and Drug Interactions (7)].
- Following a single dose of oral rolapitant, the AUC of dextromethorphan (CYP2D6 substrate) increased 2.6-fold on Day 1 and 3.3-fold on Day 8 and was similar to that following a single dose of intravenous rolapitant (VARUBI is not approved for intravenous use). Following intravenous rolapitant, inhibition of CYP2D6 continued with approximately a 3-fold increase in the AUC of dextromethorphan on Days 15 and 22 and attenuated to a 2.3-fold increase from Day 22 to Day 28, the last time point measured. See Table 6 for the summary of effects of oral and intravenous rolapitant on dextromethorphan.
|
a A single oral dose of 180 mg rolapitant was administered on Day 1; the interacting drug (dextromethorphan 30 mg) was administered orally on Day 1 with rolapitant and alone on Day 8. |
||||||||||
|
b A single intravenous dose of 166.5 mg rolapitant was administered on Day 1; the interacting drug (dextromethorphan 30 mg) was administered orally on Day 1 with rolapitant and alone on Days 8, 15, 22, and 29. VARUBI is not approved for intravenous use. The AUC of rolapitant following single intravenous dose of 166.5 mg is similar to that following single oral dose of VARUBI 180 mg. |
||||||||||
|
↑ Denotes a mean increase in exposure by the percentage indicated. |
||||||||||
|
N/A: not applicable |
||||||||||
| Rolapitant Dose (route of administration) | % Change for Dextromethorphan | |||||||||
| Day 1 with Rolapitant | Day 8 without Rolapitant | Day 15 without Rolapitant | Day 22 without Rolapitant | Day 29 without Rolapitant | ||||||
| Change in Cmax | Change in AUC | Change in Cmax | Change in AUC | Change in Cmax | Change in AUC | Change in Cmax | Change in AUC | Change in Cmax | Change in AUC | |
| 180 mga (Oral) | 120% ↑ | 160% ↑ | 180% ↑ | 230% ↑ | N/A | |||||
| 166.5 mgb (Intavenous) | 75% ↑ | 110% ↑ | 140% ↑ | 220% ↑ | 170% ↑ | 220% ↑ | 120% ↑ | 180% ↑ | 96% ↑ | 130% ↑ |
-
BCRP Transporter
-
In vitro, rolapitant is a BCRP transporter inhibitor.
- When sulfasalazine (BCRP substrate) was administered with a single oral dose of 180 mg rolapitant on Day 1 and without rolapitant on Day 8, a 140% increase in Cmax and a 130% increase in AUC of sulfasalazine 500 mg was observed on Day 1, a 17% increase in Cmax and a 32% increase in AUC was observed on Day 8 [see Drug Interactions (7)].
-
P-glycoprotein substrates
-
In vitro, rolapitant is a P-gp inhibitor.
- When digoxin (P-gp substrate) was administered with a single oral dose of 180 mg rolapitant, a 70% increase in Cmax and a 30% increase in AUC of digoxin 0.5 mg were observed [see Drug Interactions (7)].
-
Warfarin
- When warfarin was administered with a single intravenous dose of 166.5 mg rolapitant, 3% and 18% increases in Cmax and AUC of S-warfarin were observed on Day 1, respectively. On Day 8, the increases were 3% for Cmax and 21% for AUC. The effect on INR or prothrombin time was not measured. Of note, Cmax was greater with intravenous rolapitant when compared to oral VARUBI [see Drug Interactions (7)].
-
Substrates for other CYP enzymes
-
In vitro studies suggest that rolapitant is not an inhibitor of CYP1A2 and CYP2E1. In vitro studies suggest that rolapitant inhibits CYP2A6; however, a clinically meaningful drug interaction via an inhibition of CYP2A6 appears unlikely.
- No clinically significant interaction was seen on the systemic exposures of the following drugs when administered with a single oral dose of 180 mg rolapitant on Day 1: repaglinide (CYP2C8 substrate; no effect on repaglinide 0.25 mg on Day 1; on Day 8: 29% and 24% increase in Cmax and AUC, respectively), efavirenz (CYP2B6 substrate; 18% decrease in Cmax and no effect on AUC of efavirenz 600 mg on Day 1; on Day 8: no effect on Cmax and 28% increase in AUC), tolbutamide (CYP2C9 substrate; no effect on tolbutamide 500 mg on Day 1 and on Day 8), or omeprazole (CYP2C19 substrate; 44% increase in Cmax and 23% increase in AUC of omeprazole 40 mg on Day 1; on Day 8: 37% and 15% increase in Cmax and AUC, respectively).
-
Substrates for other transporters
- In vitro studies suggest that oral rolapitant is unlikely to inhibit organic anion transporting polypeptides 1B1 and 1B3 (OATP1B1 and OATP1B3), organic anion transporters 1 and 3 (OAT1 and OAT3), organic cation transporter 2 (OCT2), and multidrug and toxin extrusion proteins 1 and 2K (MATE1 and MATE2K) in vivo.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
The carcinogenic potential of rolapitant was assessed in 2-year carcinogenicity studies in CD-1 mice and Sprague-Dawley rats. In mice, there were no drug-related neoplastic findings at doses up to 135 mg/kg per day (approximately 3.6 times the recommended human dose on a body surface area basis). In rats, there were no drug-related neoplastic findings at doses up to 90 mg/kg per day (approximately 4.9 times the recommended human dose on a body surface area basis).
Mutagenesis
Rolapitant was not genotoxic in an Ames test, a human peripheral blood lymphocyte chromosome aberration test, and a mouse micronucleus test.
Impairment of Fertility
In a fertility and early embryonic development study in female rats, rolapitant at an oral dose of 9 mg/kg per day (approximately 0.5 times the recommended human dose on a body surface area basis) caused a transient decrease in maternal body weight gain and increases in the incidence of pre- and post-implantation loss. At a rolapitant dose of 4.5 mg/kg per day (approximately 0.2 times the recommended human dose on a body surface area basis), there were slight decreases in the number of corpora lutea and implantation sites. Rolapitant did not affect the fertility or general reproductive performance of male rats at doses up to 90 mg/kg per day(approximately 4.9 times the recommended human dose on a body surface area basis). Reversibility in female rats was demonstrated in a follow-up study.
14. Clinical Studies
Cisplatin-Based Highly Emetogenic Chemotherapy (HEC)
In two multicenter, randomized, double-blind, parallel group, controlled clinical studies (Study 1 and Study 2), the VARUBI regimen (VARUBI, granisetron and dexamethasone) was compared with control therapy (placebo, granisetron and dexamethasone) in patients receiving a chemotherapy regimen that included cisplatin >60 mg/m2. See Table 7 for the treatment regimens.
|
a VARUBI was administered 1 to 2 hours prior to chemotherapy treatment on Day 1. |
||
|
b Dexamethasone was administered 30 minutes prior to chemotherapy on Day 1. There is no drug interaction between VARUBI and dexamethasone, so no dosage adjustment for dexamethasone is required [see Clinical Pharmacology (12.3)]. |
||
|
c The dose of granisetron was administered 30 minutes prior to chemotherapy on Day 1. |
||
|
d VARUBI placebo was used to maintain blinding. |
||
| Day 1 | Day 2 to 4 | |
| VARUBI Regimen | ||
| Oral VARUBIa | 180 mg | None |
| Oral Dexamethasone | 20 mgb | 8 mg twice daily |
| Intravenous Granisetron | 10 mcg/kgc | None |
| Control Regimend | ||
| Oral Dexamethasone | 20 mgb | 8 mg twice daily |
| Intravenous Granisetron | 10 mcg/kgc | None |
Study 1
A total of 532 patients were randomized to either the VARUBI regimen (N=266) or control therapy (N=266). A total of 526 patients were included in the evaluation of efficacy. Of those randomized 42% were women, 58% men, 67% White, 23% Asian, 1% Black, and 9% multi-racial/other/unknown. The proportion of patients from North America was 16%. Patients in this clinical study ranged from 20 to 90 years of age, with a mean age of 57 years. In Study 1, 26% of patients were 65 years or older, with 3% of patients being 75 years or older. The mean cisplatin dose was 77 mg/m2.
During this study, 82% of the patients received a concomitant chemotherapeutic agent in addition to protocol-mandated cisplatin. The most common concomitant chemotherapeutic agents administered during Cycle 1 were: gemcitabine (17%), paclitaxel (12%), fluorouracil (11%), etoposide (10%), vinorelbine (9%), docetaxel (9%), pemetrexed (7%), doxorubicin (6%) and cyclophosphamide (5%).
Study 2
A total of 555 patients were randomized to either the VARUBI regimen (N=278) or control therapy (N=277). A total of 544 patients were included in the evaluation of efficacy. Of those randomized 32% were women, 68% men, 81% White, 14% Asian, 1% Black, and 5% multi-racial/other/unknown. The proportion of patients from North America was 7%. Patients in this clinical study ranged from 18 to 83 years of age, with a mean age of 58 years. In this study, 27% of patients were 65 years or older, with 3% of patients being 75 years or older. The mean cisplatin dose was 76 mg/m2.
During this study, 85% of the patients received a concomitant chemotherapeutic agent in addition to protocol-mandated cisplatin. The most common concomitant chemotherapeutic agents administered during Cycle 1 were: vinorelbine (16%), gemcitabine (15%), fluorouracil (12%), etoposide (11%), pemetrexed (9%), docetaxel (7%), paclitaxel (7%), epirubicin (5%) and capecitabine (4%).
The primary endpoint in both studies was complete response (defined as no emetic episodes and no rescue medication) in the delayed phase (25 to 120 hours) of chemotherapy-induced nausea and vomiting.
Moderately Emetogenic Chemotherapy (MEC) and Combinations of Anthracycline and Cyclophosphamide Chemotherapy
Study 3
In Study 3, a multicenter, randomized, double-blind, parallel group, controlled clinical study in moderately emetogenic chemotherapy, the VARUBI regimen (VARUBI, granisetron and dexamethasone) was compared with control therapy (placebo, granisetron and dexamethasone) in patients receiving a moderately emetogenic chemotherapy regimen that included at least 50% of patients receiving a combination of anthracycline and cyclophosphamide. The percentage of patients who received carboplatin in Cycle 1 was 30%. Treatment regimens for the VARUBI and control arms are summarized in Table 8.
|
a VARUBI was administered 1 to 2 hours prior to chemotherapy treatment on Day 1. |
||
|
b Dexamethasone was administered 30 minutes prior to chemotherapy on Day 1. There is no drug interaction between VARUBI and dexamethasone, so no dosage adjustment for dexamethasone is required [see Clinical Pharmacology (12.3)]. |
||
|
c The dose of granisetron was administered 30 minutes prior to chemotherapy on Day 1. |
||
|
d VARUBI placebo was used to maintain blinding. |
||
| Day 1 | Day 2 to 3 | |
| VARUBI Regimen | ||
| Oral VARUBIa | 180 mg | none |
| Oral Dexamethasone | 20 mgb | none |
| Oral Granisetron | 2 mgb | 2 mg once daily |
| Control Regimend | ||
| Oral Dexamethasone | 20 mgb | none |
| Oral Granisetron | 2 mgc | 2 mg once daily |
A total of 1369 patients were randomized to either the VARUBI regimen (N=684) or control therapy (N=685). A total of 1332 patients were included in the evaluation of efficacy. Of those randomized 80% were women, 20% men, 77% White, 13% Asian, 4% Black, and 6% multi-racial/other/unknown. The proportion of patients from North America was 33%. Patients in this clinical study ranged from 22 to 88 years of age, with a mean age of 57 years. In this study, 28% of patients were 65 years or older, with 7% of patients being 75 years or older.
The primary endpoint was complete response (defined as no emetic episodes and no rescue medication) in the delayed phase (25 to 120 hours) of chemotherapy-induced nausea and vomiting.
A summary of the study results from HEC Studies 1 and 2 and for the MEC Study 3 is shown in Table 9.
|
a Granisetron and dexamethasone were used as concomitant drugs. |
|||||||||
|
b Results were obtained based on the Cochran-Mantel-Haenszel test stratified by gender. |
|||||||||
| Endpoint | HEC Study 1 | HEC Study 2 | MEC Study 3 | ||||||
| VARUBIa (N=264) Rate (%) | Controla (N=262) Rate (%) | P-Value Treatment Difference (95% C.I.) | VARUBIa (N=271) Rate (%) | Controla (N=273) Rate (%) | P-Value Treatment Difference (95% C.I.) | VARUBIa (N=666) Rate (%) | Controla
(N=666) Rate (%) | P-Value Treatment Difference (95% C.I.) | |
| Primary Endpoint: Complete Response in the Delayed Phase | 72.7 | 58.4 | <0.001b
14.3 (6.3, 22.4) | 70.1 | 61.9 | 0.043 b
8.2 (0.3, 16.1) | 71.3 | 61.6 | <0.001b
9.8 (4.7, 14.8) |
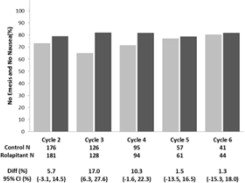
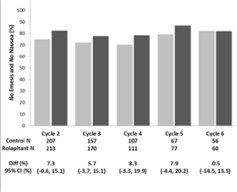
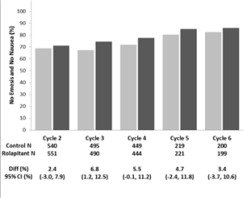
Multiple-Cycle Extension: In Studies 1, 2, and 3, patients had the option of continuing into a multiple-cycle extension for up to 5 additional cycles of chemotherapy receiving the same treatment as assigned in cycle 1. At Day 6 to 8 following initiation of chemotherapy, patients were asked to recall whether they had any episode of vomiting or retching or nausea that interfered with normal daily life. The results are summarized by study and treatment group in Figure 1 below.
Figure 1: No Emesis and No Nausea Interfering with Daily Life over Cycles 2-6
16. How is Varubi supplied
VARUBI is available as film-coated, capsule shaped, blue tablets, debossed with T0101 on one side and 100 on the other side. Each tablet contains 90 mg rolapitant. VARUBI is packaged in an Aclar blister shell with aluminum foil backing and supplied as follows:
| NDC 70720-101-02 | A single dose child-resistant wallet (2 tablets as one set of twinned blisters) |
Store at 20ºC to 25ºC (68ºF to 77ºF); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Drug Interactions
Advise patients to tell their healthcare provider when they start or stop taking any concomitant medications. VARUBI is a moderate CYP2D6 inhibitor and can increase plasma concentrations of CYP2D6 substrates [see Contraindications (4), Warnings and Precautions (5.1), Drug Interactions (7)].
Infertility
Advise females of reproductive potential that VARUBI may impair fertility [see Use in Specific Populations (8.3), Nonclinical Toxicology (13.1)].
Manufactured for: TerSera Therapeutics LLC, Deerfield, IL 60015
©2020 TerSera Therapeutics LLC
|
This Patient Information has been approved by the U.S. Food and Drug Administration. |
Revised: 8/2020 |
| PATIENT INFORMATION VARUBI® (vuh ROO bee) (rolapitant) tablets, for oral use |
|
What is VARUBI?
|
|
Do not take VARUBI if you:
|
|
Before taking VARUBI, tell your doctor about all of your medical conditions, including if you:
|
|
| Tell your doctor about all the medicines you take or stop taking, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Keep a list of your medicines to show your doctor and pharmacist when you get a new medicine. VARUBI and other medicines may affect each other and could cause serious side effects. |
|
How should I take VARUBI?
|
|
| What are the possible side effects of VARUBI?
VARUBI may cause serious side effects, including:
|
|
| The most common side effects of VARUBI in people who take VARUBI and receive Cisplatin chemotherapy medicine include: low white blood cell count, hiccups, and stomach (abdominal) pain. The most common side effects of VARUBI in people who take VARUBI and receive Anthracycline and Cyclophosphamide chemotherapy medicines include: decreased appetite, low white blood cell count, dizziness, indigestion, urinary tract infection, mouth sores, and low red blood cell count. These are not all the possible side effects of VARUBI. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|
How should I store VARUBI?
|
|
| Keep VARUBI and all medicines out of the reach of children. | |
| General information about the safe and effective use of VARUBI.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use VARUBI for a condition for which it was not prescribed. Do not give VARUBI to other people, even if they have the same symptoms that you have. It may harm them. You can ask your doctor or pharmacist for information about VARUBI that is written for health professionals. |
|
| What are the ingredients in VARUBI?
Active ingredient: rolapitant Inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, povidone, and pregelatinized starch. The tablets are coated in non-functional blue and clear coats. The tablet coating comprises the following inactive ingredients: FD&C Blue No. 2-Indigo Carmine Lake, polyethylene glycol, polysorbate 80, polyvinyl alcohol, talc, and titanium dioxide. Manufactured for: TerSera Therapeutics LLC, Deerfield, IL 60015 For more information, go to www.varubirx.com or call 1-844-334-4035. |
|
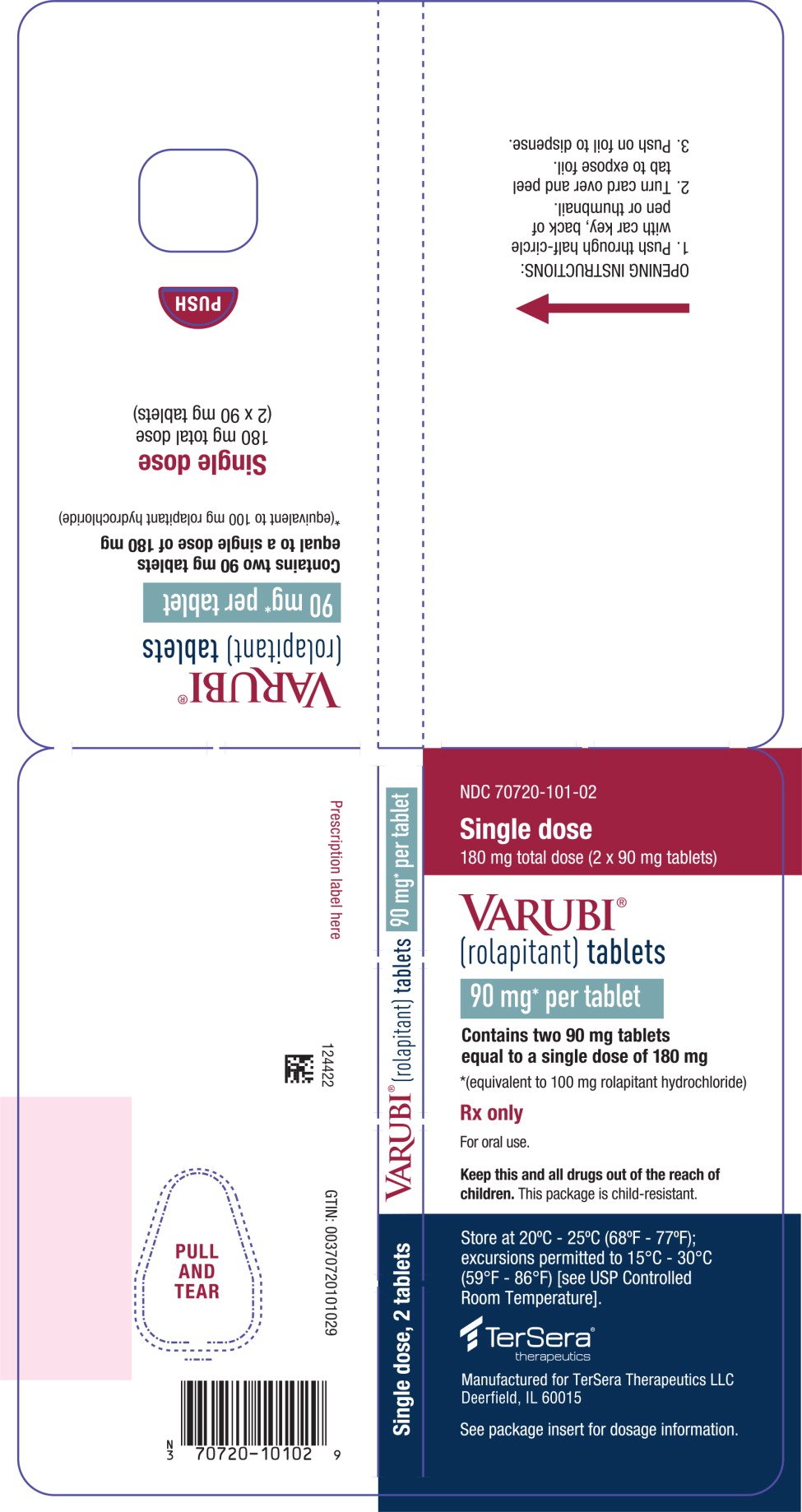
PRINCIPAL DISPLAY PANEL - 90 mg Tablet Blister Wallet
NDC 70720-101-02
Single dose
180 mg total dose (2 x 90 mg tablets)
VARUBI®
(rolapitant) tablets
90 mg* per tablet
Contains two 90 mg tablets
equal to a single dose of 180 mg
*(equivalent to 100 mg rolapitant hydrochloride)
Rx only
For oral use.
Keep this and all drugs out of the reach of
children. This package is child-resistant.
Store at 20°C - 25°C (68°F - 77°F);
excursions permitted to 15°C - 30°C
(59°F - 86°F) [see USP Controlled
Room Temperature].
TerSera®
therapeutics
Manufactured for TerSera Therapeutics LLC
Deerfield, IL 60015
See package insert for dosage information.
| VARUBI
rolapitant tablet |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - TerSera Therapeutics LLC (080226115) |
Frequently asked questions
More about Varubi (rolapitant)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (1)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- Drug class: NK1 receptor antagonists
- Breastfeeding
- En español
Patient resources
- Varubi drug information
- Varubi (Rolapitant Intravenous) (Advanced Reading)
- Varubi (Rolapitant Oral) (Advanced Reading)