Nitro-Time: Package Insert / Prescribing Info
Package insert / product label
Generic name: nitroglycerin
Dosage form: extended-release capsules
Drug classes: Antianginal agents, Vasodilators
Medically reviewed by Drugs.com. Last updated on Mar 3, 2025.
On This Page
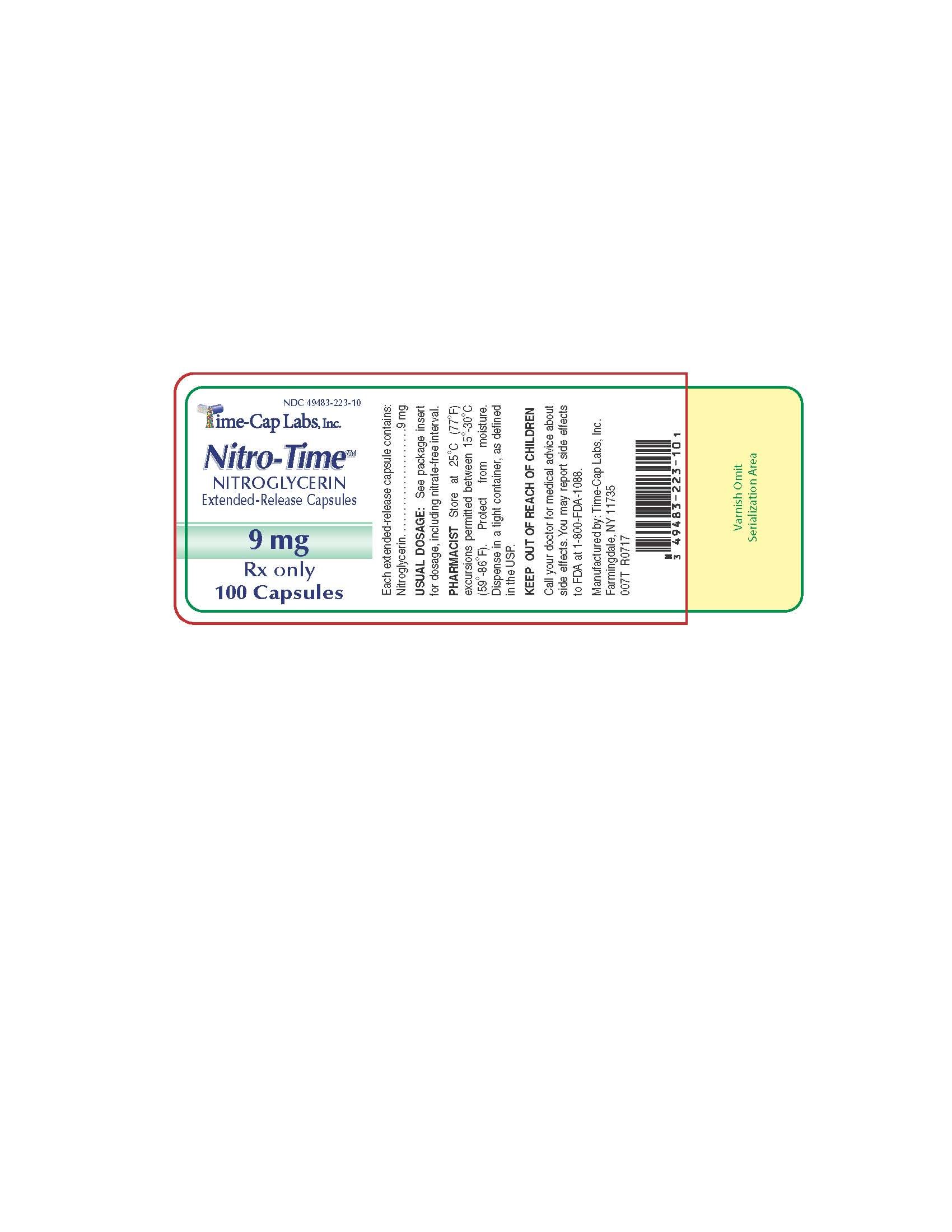
How is Nitro-Time supplied
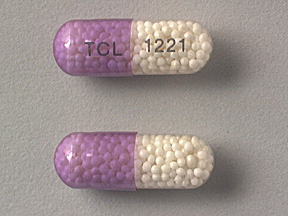
2.5 mg Nitroglycerin Extended-Release (Pink and Clear Capsules imprinted TCL-1221) in bottles of 60's & 100's
How is Nitro-Time supplied
6.5 mg Nitroglycerin Extended-Release Capsules Blue/Yellow imprinted TCL1222 in bottles of 60's and 100's
| NITRO-TIME
nitroglycerin capsule |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| NITRO-TIME
nitroglycerin capsule |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| NITRO-TIME
nitroglycerin capsule |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Labeler - TIME CAP LABORATORIES, INC (037052099) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| TIME CAP LABORATORIES, INC | 037052099 | manufacture(49483-221, 49483-222, 49483-223) | |
Frequently asked questions
- How do you take GoNitro to treat an angina attack (chest pain)?
- What is the shelf life of nitroglycerin tablets?
More about Nitro-Time (nitroglycerin)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Drug class: antianginal agents
- Breastfeeding
- En español
Patient resources
Professional resources
Other brands
Nitrostat, Nitro-Bid, Nitrolingual Pumpspray, Rectiv, ... +4 more