Minitran: Package Insert / Prescribing Info
Package insert / product label
Generic name: nitroglycerin
Dosage form: patch
Drug classes: Antianginal agents, Vasodilators
Medically reviewed by Drugs.com. Last updated on Jan 20, 2025.
MINITRAN™ 5 mg (Italy)
MINITRAN™ 5 mg/24 ore
Cerotti transdermici
AIC n. 027028012
Nitroglicerina
15 cerotti transdermici
superficia di rilascio:
6,7 cm2
MEDA
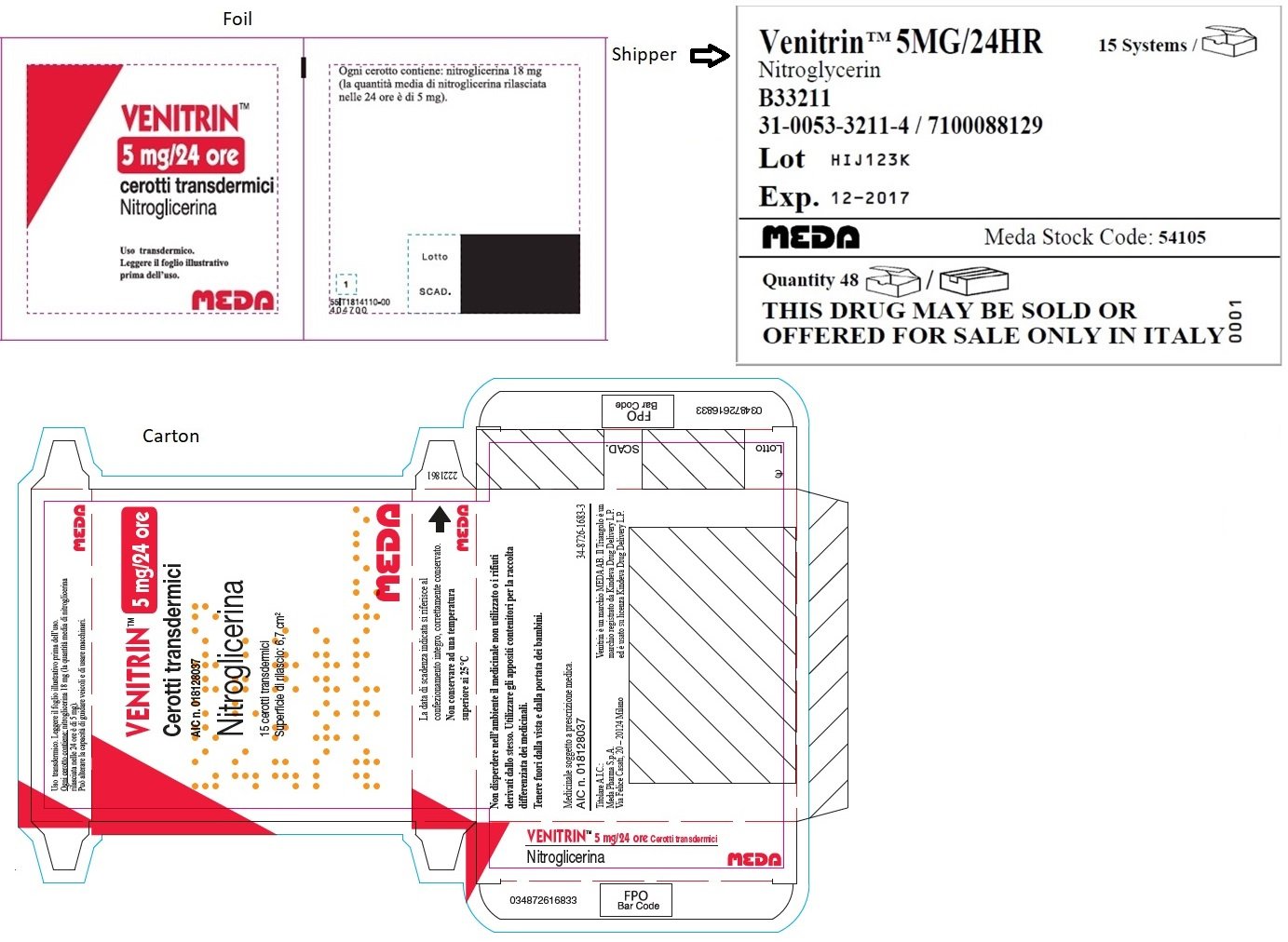
VENITRAN™ 5 mg (Italy)
VENITRAN™ 5MG/24HR
Nitroglycerin
B33211
31-0053-3211-4 / 7100088129
Lot HIJ123K
Exp. 12-2017

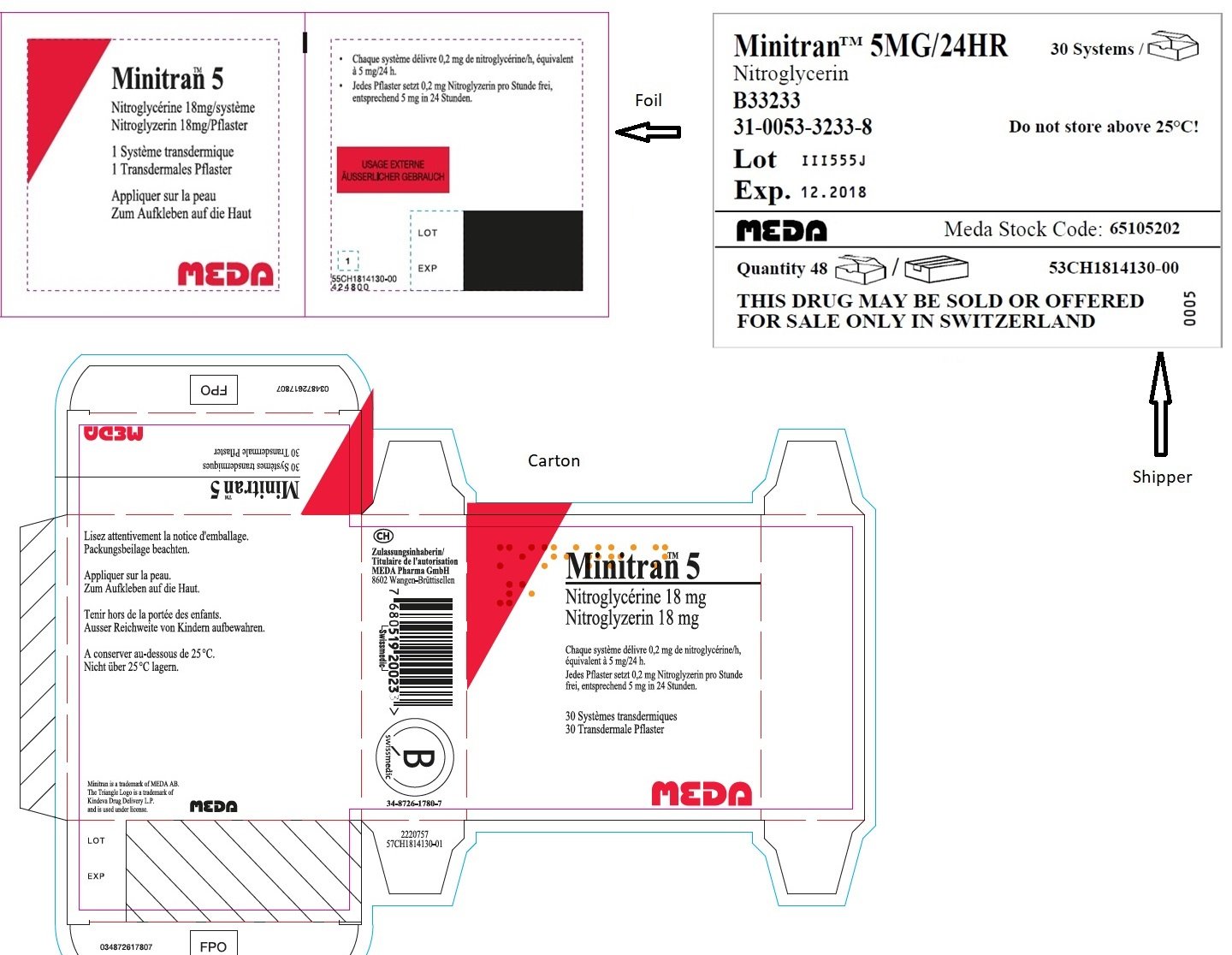
MINITRAN™ 5 mg (Switzerland)
MINITRAN™ 5
Nitroglycerin
B33233
31-0053-3233-8
Lot III555J
Exp. 12.2018
MEDA
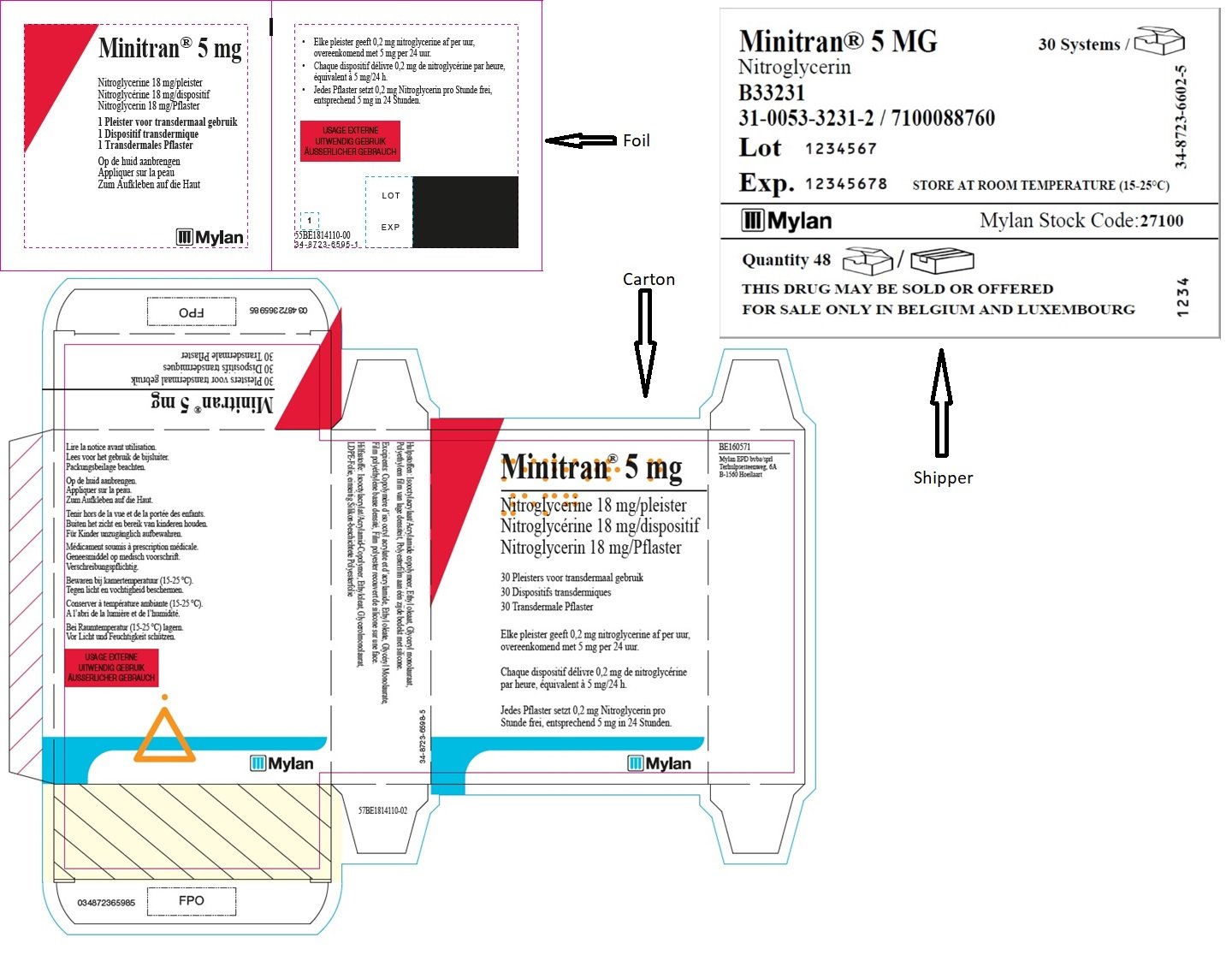
MINITRAN™ 5 mg (Belgium)
MINITRAN™ 5 mg
Nitroglycerine 18 mg/pleister
Nitroglycérine 18 mg/dispositif
Nitroglycerin 18 mg/pflaster
30 Pleisters voor transdermaal gebruik
30 Dispositifs transdermiques
30 Transdermale Pflaster
Elke pleister geeft 0,2 mg nitroglycerine af per uur,
overeenkomend met 5 mg per 24 uur.
Chaque dispositif délivre 0,23 mg de nitroglycérine
par heure, équivalent à 5 mg/24 h.
Jedes Pflaster setzt 0,2 mg Nitroglycerin pro
Stunde frei, entsprechend 5 mg in 24 Stunden.
MYLAN
MINITRAN™ 5 mg (Mexico)
MINITRAN™
Trinitrato de glicerilo
31-0053-3204-9
Lote: 999991A
Cad: MAR18
Reg. No. 074M91 SSA IV
Parche 18 mg
Cada parche libera 0.2 mg por hora. 5 mg/24 horas
Hecho en EUA por:
3M Drug Delivery Systems
Northridge CA, 91324, EUA
Importado y Distribuido por:
More Pharma Corporation S. de R.L. de C.V.
Blvd. Adolfo Lopez Mateos No. 314 PB, Col.
Tiacopac, C.P. 01049
Deleg. Alvaro Obregón
Ciudad de México, México
More Pharma
Pharmaceutical Company
Consérvese a temperatura ambiente a no mas de
30ºC y en lugar seco.
Quantity 5760

MINITRAN™ 10 mg (Italy)
MINITRAN™ 10 mg/24 ore
Cerotti transdermici
AIC n. 027028024
Nitroglicerina
15 cerotti transdermici
Superficie di rilascio:
13,3 cm 2
MEDA

VENITRAN™ 10 mg (Italy)
VENITRAN™ 10MG/24HR
Nitroglycerin
B33333
31-0053-3333-6
Lot KKK666L
Exp. 12.2018
MEDA

Related/similar drugs
MINITRAN® 10 mg (Belgium)
MINITRAN® 10 mg
Nitroglycerine 36 mg/pleister
Nitroglycérine 36 mg/dispositif
Nitroglycerin 36 mg/pflaster
30 Pleisters voor transdermaal gebruik
30 Dispositifs transdermiques
30 Transdermale Pflaster
Elke pleister geeft 0,4 mg nitroglycerine af per uur,
overeenkomend met 10 mg per 24 uur.
Chaque dispositif délivre 0,4 mg de nitroglycérine
par heure, équivalent à 10mg/24 h.
Jedes Pflaster setzt 0,4 mg Nitroglycerin pro
Stunde frei, entsprechend 10 mg in 24 Stunden.
MYLAN
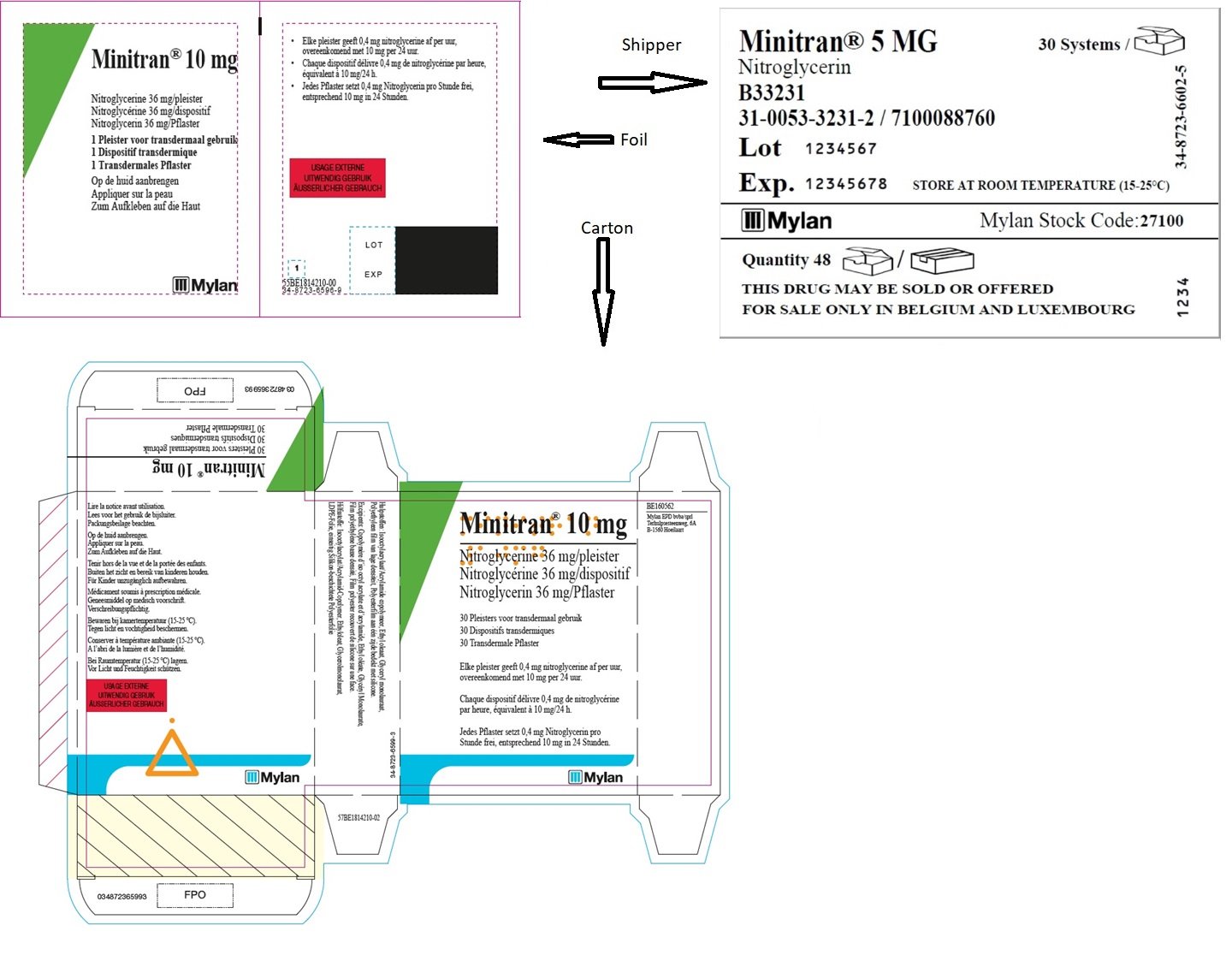
MINITRAN™ 10 (Switzerland)
MINITRAN™ 10MG/24HR
Nitroglycerin
B33333
31-0053-3333-6
Lot KKK666L
Exp. 12.2018
MEDA
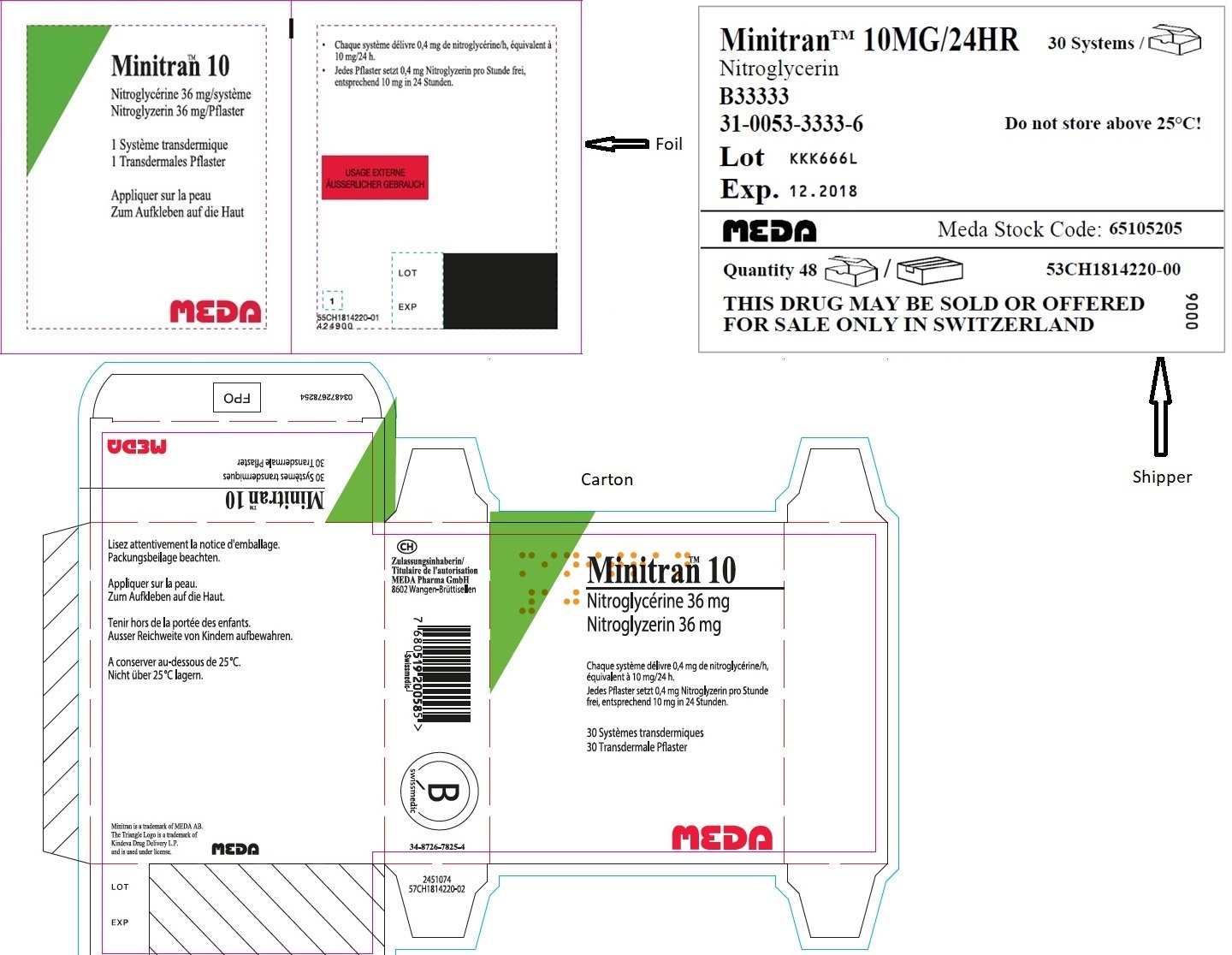
MINITRAN™ 15 mg (Italy)
MINITRAN™ 15 mg/24 ore
Cerotti transdermici
AIC n. 027028036
Nitroglicerina
15 cerotti transdermici
Superficie di rilascio: 20 cm 2
MEDA
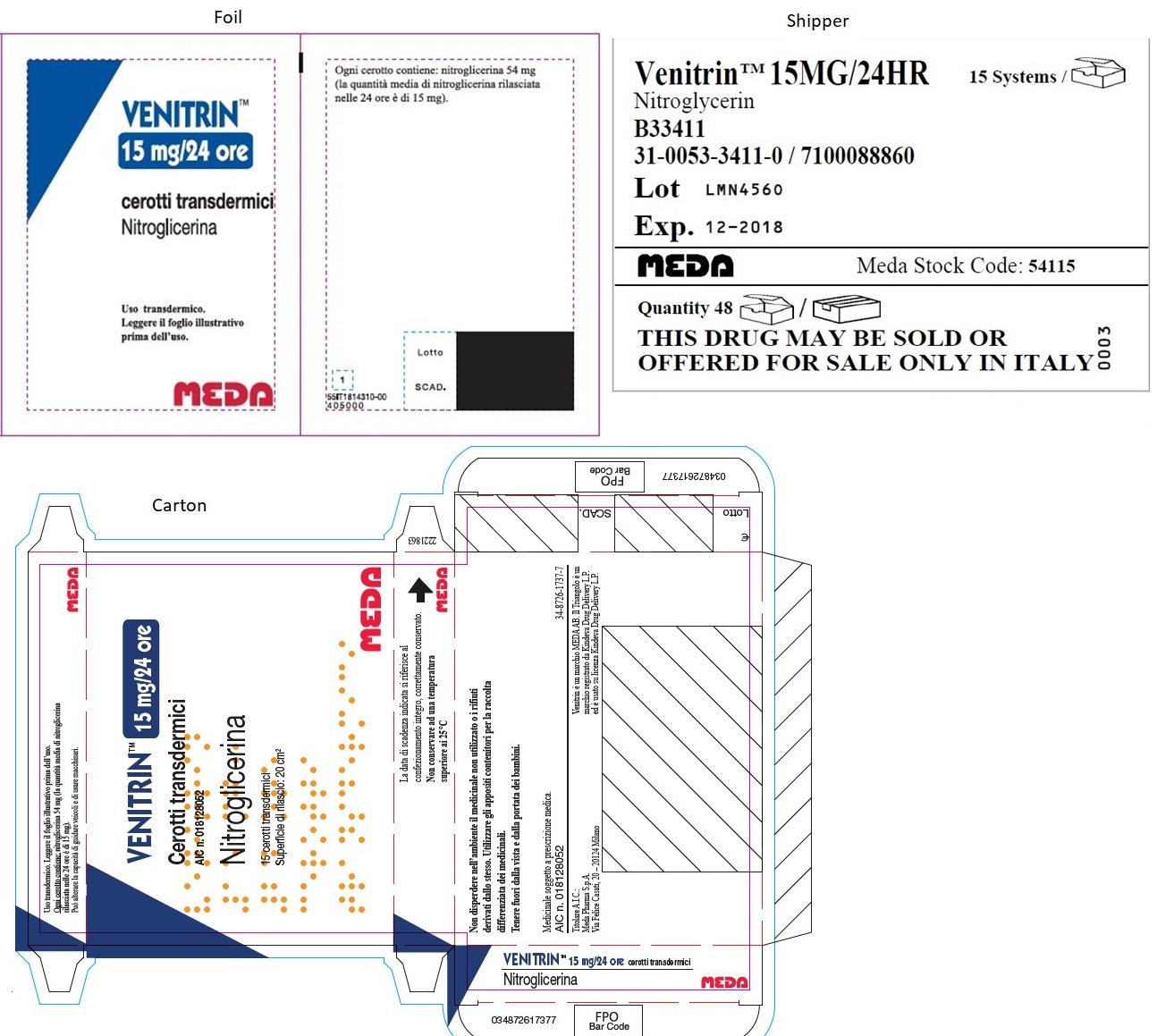
VENITRAN™ 15 mg (Italy)
VENITRAN™ 15MG/24HR
Nitroglycerin
B33411
31-0053-3411-0 / 7100088860
Lot LMN4560
Exp. 12-2018
MEDA

MINITRAN™ 15 mg (Belgium)
MINITRAN™ 15 mg
Nitroglycerine 54 mg/pleister
Nitroglycérine 54 mg/dispositif
Nitroglycerin 54 mg/pflaster
30 Pleisters voor transdermaal gebruik
30 Dispositifs transdermiques
30 Transdermale Pflaster
Elke pleister geeft 0,6 mg nitroglycerine af per uur,
overeenkomend met 15 mg per 24 uur.
Chaque dispositif délivre 0,6 mg de nitroglycérine
par heure, équivalent à 15 mg/24 h.
Jedes Pflaster setzt 0,6 mg Nitroglycerin pro
Stunde frei, entsprechend 15 mg in 24 Stunden.
MYLAN

MINITRAN™ 10 mg (Mexico)
MINITRAN™
Trinitrato de Glicerilo
Parche
36 mg
Cada Parche Libera 0.4 mg por hora
10 mg/24 horas
Lote/Lot: XXXXXX
Cad./Exp.: YYYXX
5760
Consérvese a temperatura
ambiente a no más de
30° yen en lugar seco.
Reg. No. 074M91 SSA IV
T M: Marca Registrada/Registered Trademark
More Pharma
Pharmaceutical Company
31-0053-3304-7
Distribuido Por:
More Pharma Corporation S. De R.L.. De C.V.
Av. Ejército Nacional No. 926
Col. Los morales, Seccion Palmas
C.P. 11540, Dele. Miguel Hidalgo,
D.F., México.
Hecho en E.U.A Por:
3M Drug Delivery Systems,
Northridge, Ca 91324 EUA

| MINITRAN
nitroglycerin patch |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| MINITRAN
nitroglycerin patch |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| MINITRAN
nitroglycerin patch |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Kindeva Drug Delivery L.P. (117492677) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Kindeva Drug Delivery L.P. | 128688199 | manufacture(0089-0171, 0089-0172, 0089-0173) | |
Frequently asked questions
- How do you take GoNitro to treat an angina attack (chest pain)?
- What is the shelf life of nitroglycerin tablets?
More about Minitran (nitroglycerin)
- Check interactions
- Compare alternatives
- Side effects
- Dosage information
- During pregnancy
- Drug class: antianginal agents
- Breastfeeding
Patient resources
Professional resources
Other brands
Nitrostat, Nitro-Bid, Nitrolingual Pumpspray, Rectiv, ... +4 more
