Supprelin LA: Package Insert / Prescribing Info
Package insert / product label
Generic name: histrelin acetate
Dosage form: subcutaneous implant
Drug classes: Gonadotropin releasing hormones, Hormones / antineoplastics
J Code (medical billing code): J9225 (50 mg)
Medically reviewed by Drugs.com. Last updated on Apr 21, 2025.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Drug Interactions
- Use In Specific Populations
- Overdosage
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Patient Counseling Information
- Medication Guide
Highlights of Prescribing Information
SUPPRELIN LA (histrelin acetate) subcutaneous implant
Initial U.S. Approval: 1991
Recent Major Changes
Warnings and Precautions (5.5) 04/2022
Indications and Usage for Supprelin LA
SUPPRELIN LA is a gonadotropin releasing hormone (GnRH) agonist indicated for the treatment of children with central precocious puberty (CPP) (1).
Supprelin LA Dosage and Administration
The recommended dose of SUPPRELIN LA is one implant every 12 months. The implant is inserted subcutaneously in the inner aspect of the upper arm and provides continuous release of histrelin for 12 months of hormonal therapy (2).
Dosage Forms and Strengths
SUPPRELIN LA is available as a 50 mg histrelin acetate subcutaneous implant which delivers approximately 65 mcg histrelin acetate per day over 12 months (3).
Contraindications
Warnings and Precautions
- Initial Agonistic Action: Initial transient increases of estradiol and/or testosterone may cause a temporary worsening of symptoms (5.1).
- Implant Breakage: Have been observed during implant removal. Monitor luteinizing hormone, follicle stimulating hormone or testosterone for suppression of CPP (5.2, 5.6)
- Psychiatric Events: Have been reported in patients taking GnRH agonists. Events include emotional lability, such as crying, irritability, impatience, anger, and aggression. Monitor for development or worsening of psychiatric symptoms (5.3).
- Convulsions: Have been observed in patients receiving GnRH agonists with or without a history of seizures, epilepsy, cerebrovascular disorders, central nervous system anomalies or tumors, and in patients on concomitant medications that have been associated with convulsions (5.4).
- Pseudotumor Cerebri (Idiopathic Intracranial Hypertension): Have been reported in pediatric patients receiving GnRH agonists. Monitor patients for headache, papilledema, and blurred vision. (5.5)
Adverse Reactions/Side Effects
- The most common adverse reaction is implant site reaction (51.1%), including complications related to the insertion or removal of the implant (6).
- Adverse events related to suppression of endogenous sex steroid secretion may occur (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Endo Pharmaceuticals Solutions Inc. at 1-800-462-3636 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
Use In Specific Populations
Use of SUPPRELIN LA in children less than 2 years of age is not recommended (8.4).
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 4/2022
Full Prescribing Information
1. Indications and Usage for Supprelin LA
SUPPRELIN LA (histrelin acetate) subcutaneous implant is indicated for the treatment of children with central precocious puberty (CPP).
Children with CPP (neurogenic or idiopathic) have an early onset of secondary sexual characteristics (earlier than 8 years of age in females and 9 years of age in males). They also show a significantly advanced bone age that can result in diminished adult height attainment.
Prior to initiation of treatment a clinical diagnosis of CPP should be confirmed by measurement of blood concentrations of total sex steroids, luteinizing hormone (LH) and follicle stimulating hormone (FSH) following stimulation with a GnRH analog, and assessment of bone age versus chronological age. Baseline evaluations should include height and weight measurements, diagnostic imaging of the brain (to rule out intracranial tumor), pelvic/testicular/adrenal ultrasound (to rule out steroid secreting tumors), human chorionic gonadotropin levels (to rule out a chorionic gonadotropin secreting tumor), and adrenal steroids to exclude congenital adrenal hyperplasia.
2. Supprelin LA Dosage and Administration
2.1 Recommended Dose
The recommended dose of SUPPRELIN LA is one implant every 12 months. Each implant contains 50 mg histrelin acetate. The implant is inserted subcutaneously in the inner aspect of the upper arm and provides continuous release of histrelin acetate (65 mcg/day) for 12 months of hormonal therapy. SUPPRELIN LA should be removed after 12 months of therapy (the implant has been designed to allow for a few additional weeks of histrelin acetate release, in order to allow flexibility of medical appointments). At the time an implant is removed, another implant may be inserted to continue therapy. Discontinuation of SUPPRELIN LA should be considered at the discretion of the physician and at the appropriate time point for the onset of puberty (approximately 11 years for females and 12 years for males).
2.2 Recommended Procedure for Implant Insertion and Removal
This procedure section is intended to provide guidance for the insertion and removal of SUPPRELIN LA. The actual procedure used, however, is at the discretion of the qualified healthcare provider performing the procedure.
Insertion of a new implant can proceed using the following Suggested Insertion Procedure. If a previous SUPPRELIN LA implant must first be removed, please see the Suggested Removal Procedure instructions below.
Suggested Insertion Procedure
The supplies necessary to insert the implant, including the Insertion Tool and local anesthetic, are provided in a separate Implantation Kit that is shipped along with the implant. Please note that the implant should be kept refrigerated (2-8°C) in its sealed vial, pouch, and carton, until needed for the procedure. Once removed from refrigeration, the vial containing the implant (still in its unopened pouch and carton) may remain at room temperature for up to 7 days, if necessary, before being used. If not used in that time, the packaged implant may again be properly refrigerated until the expiration date on the carton.
NOTE: The Implantation Kit is to be stored at room temperature and should not be refrigerated.
Insertion of the SUPPRELIN LA implant is a surgical procedure. Sterile gloves and aseptic technique must be used to minimize any chance of infection.
Setting up the Sterile Field
Using proper aseptic technique, the sterilized components of the Implantation Kit needed for the insertion procedure, including the Insertion Tool, are to be carefully dispensed from their packaging onto the Sterile Field drape (non-fenestrated) provided. NOTE THAT THE KIT BOX AND ALL PACKAGING ARE NOT STERILE and should be kept off of the Sterile Field drape. DO NOT PLACE THE VIAL OF LOCAL ANESTHETIC OR THE VIAL CONTAINING THE IMPLANT ONTO THE DRAPE as the exterior surface of these vials is not sterile.
The implant vial should not be opened until just before the time of insertion. Open the vial by removing the metal band and carefully pour the sterile contents (implant and sterile saline) onto the Sterile Field drape. The implant can then be handled with sterile gloves or with the sterile mosquito clamp provided. AVOID bending or pinching the implant.
Preparing the Patient and the Insertion Site
The patient should be on his/her back, ideally with the arm least used (e.g., left arm for a right-handed person) positioned, either bent or extended, so that the physician has ready access to the inner aspect of the upper arm. Propping the arm with pillows may help the patient more easily hold the position. The suggested optimum site for subcutaneous insertion is approximately half-way between the shoulder and the elbow, in line with the crease between the biceps and triceps muscles.
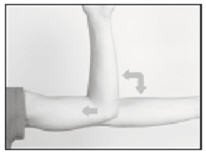
Antiseptic
Swab the insertion area with topical antiseptic, then overlay with the fenestrated Sterile Field drape provided, so that the opening is over the insertion site (for clarity of illustration, the following images do not show the drape).
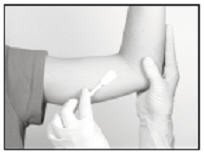
Anesthetic
The method of anesthesia utilized (i.e., local, conscious sedation, general) is at the discretion of the healthcare provider.
If local anesthesia is selected: a vial of sterile local anesthetic (note that the exterior of the vial is not sterile) has been provided along with a sterile hypodermic needle for injection. After determining the absence of known allergies to the anesthetic agent, inject anesthetic into the subcutaneous tissue, starting at the planned incision site, then infiltrating along the intended subcutaneous insertion path, up to the length of the implant (a little more than one inch). Local anesthesia may also be supplemented by the use of distraction techniques.
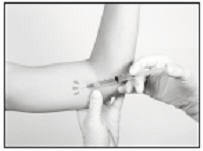
The following sections describe the suggested procedure for insertion of the implant using the Insertion Tool provided. The method of insertion used, however, is at the discretion of the healthcare provider performing the procedure.
Loading the Insertion Tool
The sterile Insertion Tool is comprised of a fixed handle attached to a retractable, bevel-tipped cannula, into the chamber of which the implant is to be placed for subcutaneous insertion. The cannula can be extended and retracted. The fully extended cannula contains a fixed piston upon which the implant, once inserted, rests. During the final step of the insertion procedure, the cannula will be retracted into the handle using the slide mechanism (green button), thereby exposing and leaving the implant to remain in the subcutaneous tissue.
When first grasping the sterile Insertion Tool, confirm that the cannula is fully extended. Verify this by inspecting the position of the green retraction button. The button should be locked in position all the way forward, towards the cannula, farthest from the handle.

The implant can be picked up using sterile gloves or with the sterile mosquito clamp provided. Avoid bending or pinching the implant. Note that the implant may come out of its vial slightly curved and/or partially flattened after refrigerated storage. To help make the implant more symmetrical prior to loading into the Tool, you can roll the implant a few times (while wearing a sterile glove) between the fingers and thumb.
Insert the implant into the cannula of the Insertion Tool manually or using the mosquito clamp. When inserting the implant into the cannula, DO NOT FORCE the implant. If resistance is felt, the implant should be removed and manually manipulated or rolled as needed, and re-inserted into the cannula.
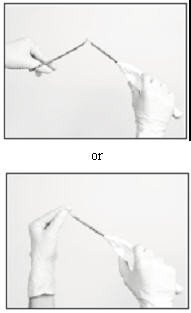
When fully inserted, the implant rests inside the cannula so that just the tip of the implant is visible at the beveled end of the cannula.
Making the Incision
Using the sterile scalpel provided, make an incision transverse to the long axis of the arm, and of a size adequate to allow the bore of the cannula to be inserted into the subcutaneous tissue. Be sure that the incision is positioned so that there is sufficient length of upper arm available to fit the implant easily within the intended insertion space.
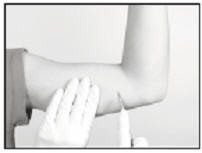
Inserting the Implant
It is suggested that insertion may be easier if a “pocket” for the implant is first created by blunt dissection through the incision, subcutaneously along the path of the anesthetic, using the cannula of the loaded Insertion Tool, or using a sterile hemostatic clamp or equivalent surgical tool.
Be sure to VISIBLY RAISE THE SKIN (known as tenting) at all times during the pocket-making and insertion procedures to ensure correct subcutaneous placement (“just under the skin”) of the implant. Note that the cannula of the Insertion Tool, or whatever tool is being used to create the pocket, SHOULD NOT ENTER MUSCLE TISSUE. Deep insertion of the implant will not affect the performance of SUPPRELIN LA, but may cause difficulty in the later removal of the implant.
If using the cannula of the loaded Insertion Tool to create the pocket, carefully insert the tip of the cannula into the incision and advance through the subcutaneous tissue, while visibly raising the skin along the length of the cannula up to, but no farther than, the inscribed black line on the cannula. DO NOT DEPRESS THE GREEN RETRACTION BUTTON ON THE TOOL WHILE INSERTING OR ADVANCING THE TOOL INTO THE INCISION.
Pull the Tool back, almost to the beveled tip of the cannula, and advance the Tool forward again, so that the cannula re-enters the pocket completely, but no farther than the inscribed black line. Be sure to keep the insertion path just immediately subcutaneous.
If another tool was used to create the pocket, now insert the loaded cannula of the Insertion Tool containing the implant through the incision, up to the inscribed black line.
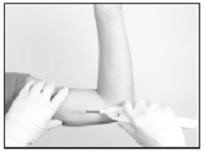
Hold the Insertion Tool in place with the base against the patient’s arm (if possible) as you carefully move your thumb to the green retraction button. Depress the button to release the locking mechanism, then slide the button back toward the handle until it stops, all the while holding the body of the Insertion Tool in place.
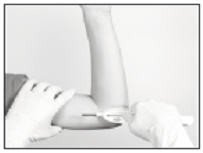
Retracting the button causes the cannula to withdraw from the incision, leaving the implant in the subcutaneous tissue. DO NOT FURTHER ADVANCE THE CANNULA ONCE THE RETRACTION PROCESS HAS STARTED. Likewise, do not withdraw the Insertion Tool until the button is fully retracted or the implant may be pulled partially out of the incision. Once the retraction is complete, the Tool can be fully withdrawn.
NOTE: It may be helpful during the process of retraction and withdrawal of the cannula to apply pressure to the skin over the implant, to help ensure that the implant remains in the subcutaneous pocket.
If there is a need to re-start the process at any time during the insertion procedure, withdraw the Insertion Tool, carefully extract the implant from the cannula and reset the retraction button on the Tool to its forward-most position. Examine the implant before reloading the implant into the Insertion Tool, and start again.
Placement of the implant should be confirmed by palpation. Note that the tip of a properly-placed implant may not be visible through the incision.
After implantation, briefly cover the site with a sterile gauze pad and apply pressure to ensure hemostasis.
Closing the Incision
To close the incision, you can use the absorbable sutures and/or the sterile adhesive surgical strips provided. To improve adhesion of the strips, you can apply benzoin tincture antiseptic (provided) to the skin, and let it dry, before applying the adhesive strips.

Once closed, cover the incision site with sterile gauze pads and secure the dressing with the bandage provided.
Please provide the patient’s parent or guardian with a Patient Information Leaflet, which includes information about the implant and instructions on proper care of the insertion site.
Suggested Removal Procedure
SUPPRELIN LA should be removed after 12 months of therapy. Most of the supplies necessary to remove the implant, including the local anesthetic and the sterile mosquito clamp, are provided in the Implantation Kit that is shipped along with a new SUPPRELIN LA implant. Note that the Implantation Kit is to be stored at room temperature and must not be refrigerated. See the Suggested Insertion Procedure above for further instructions.
Removal of the SUPPRELIN LA implant is a surgical procedure. Sterile gloves and aseptic technique must be used to minimize any chance of infection.
Setting up the Sterile Field
Using proper aseptic technique, the sterilized components of the Implantation Kit needed for the implant removal procedure are to be carefully dispensed from their packaging out onto the Sterile Field drape (non-fenestrated) provided. NOTE THAT THE KIT BOX AND ALL PACKAGING ARE NOT STERILE and should be kept off of the Sterile Field drape. DO NOT PLACE THE VIAL OF LOCAL ANESTHETIC ONTO THE DRAPE as the exterior surface of the vial is not sterile.
Preparing the Patient and the Site
The patient should be on his/her back, with the arm containing the implant positioned, either bent or extended, so that the physician has ready access to the inner aspect of the upper arm. Propping the arm with pillows may help the patient more easily hold the position.
The implant to be removed should first be located by palpating the inner aspect of the upper arm, near the incision from the prior year.
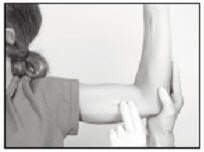
Generally, the previous implant is readily palpated. In the event the implant is difficult to locate, ultrasound may be used. If ultrasound fails to locate the implant, other imaging techniques such as CT or MRI may be used to locate it (plain films are not recommended as the implant is not radiopaque).
Antiseptic
Swab the area above and around the previous implant with topical antiseptic. Overlay the area with the fenestrated Sterile Field drape provided, so that the hole is over the previous insertion site (for clarity of illustration, the following images do not show the drape).
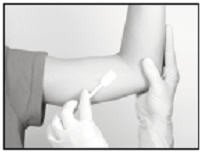
Anesthetic
The method of anesthesia utilized (i.e., local, conscious sedation, general) is at the discretion of the healthcare provider.
If local anesthesia is selected: a vial of sterile local anesthetic (note that the exterior of the vial is not sterile) has been provided along with a sterile hypodermic needle for injection. After determining the absence of known allergies to the anesthetic agent, inject anesthetic into the subcutaneous tissue at and around the site of the intended incision (the site of the previous implant). Local anesthesia may also be supplemented by the use of distraction techniques.
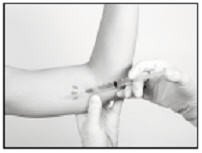
Making the Incision and Removing the Implant
Using the sterile scalpel provided, make an incision of a size adequate to allow the implant to be easily removed and, if a new implant will be inserted, large enough for the bore of the cannula of the Insertion Tool provided.
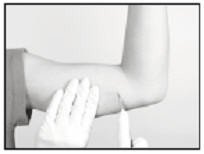
Generally, the tip of the implant will be visible through the incision, possibly covered by a pseudocapsule of tissue. In order to facilitate the removal of the implant, it may be necessary to palpate the head of the implant through the incision using your smallest finger, especially if the head of the implant is not readily visible. In addition, you may need to push down on the distal end of the implant and “massage it forward” towards the incision.
Carefully nick the pseudocapsule to reveal the polymer tip of the implant. It may be beneficial to insert the sterile mosquito clamp provided into the hole created in the pseudocapsule and expand by opening the clamp. Widening the opening of the pseudocapsule may ease the extraction of the implant.
Gently but securely grasp the implant with the sterile mosquito clamp and extract the implant.
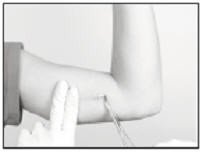
Dispose of the implant in a proper manner, treating it like any other bio-waste.
Briefly cover the site with a sterile gauze pad and apply pressure to ensure hemostasis.
If inserting a new implant, see the Suggested Insertion Procedure instructions provided above. Note that you can insert the new implant into the same “pocket” as the removed implant, or make a new incision at a different site in the same arm or in the contralateral arm.
If a new implant is not to be inserted, proceed to close the incision.
Closing the Incision
To close the incision, you can use the absorbable sutures and/or the sterile adhesive surgical strips provided. To improve adhesion of the strips, you can apply benzoin tincture antiseptic (provided) to the skin, and let it dry, before applying the adhesive strips.
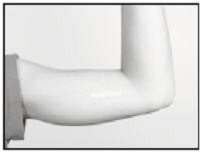
Once closed, cover the incision site with sterile gauze pads and secure the dressing with the bandage provided.
3. Dosage Forms and Strengths
SUPPRELIN LA is a sterile, nonbiodegradable, diffusion-controlled, hydrogel polymer reservoir drug delivery system designed to deliver histrelin acetate continuously for 12 months after subcutaneous implantation. The sterile implant contains 50 mg histrelin acetate and delivers approximately 65 mcg histrelin acetate per day over 12 months.
4. Contraindications
SUPPRELIN LA is contraindicated in:
- Patients who are hypersensitive to gonadotropin releasing hormone (GnRH) or GnRH agonist analogs
- Pregnancy [see Use in Specific Populations (8.1)].
5. Warnings and Precautions
5.1 Initial Agonistic Action
SUPPRELIN LA, like other GnRH agonists, initially causes a transient increase in serum concentrations of estradiol in females and testosterone in both sexes during the first week of treatment. Patients may experience worsening of symptoms or onset of new symptoms during this period. However, within 4 weeks of histrelin therapy, suppression of gonadal steroids occurs and manifestations of puberty decrease.
5.2 Implant Breakage
Implant insertion is a surgical procedure and it is important that the insertion instructions are followed to avoid potential complications. The insertion and removal of the implant should be done aseptically. Proper surgical technique is critical in minimizing adverse events related to the insertion and the removal of the histrelin implant. On occasion, localizing and/or removal of implant products have been difficult and imaging techniques were used, including ultrasound, CT, or MRI (note: the histrelin implant is not radiopaque). In some cases the implant broke during removal and multiple pieces were recovered. Confirm that the entire implant has been removed. If the implant was not retrieved completely, the remaining pieces should be removed following the instructions in the Suggested Removal Procedure section [see Dosage and Administration (2.2 )]. Rare events of spontaneous extrusion of the implant have been observed in clinical trials. During SUPPRELIN LA treatment, patients should be evaluated for evidence of clinical and biochemical suppression of CPP manifestations (see Section 5.6, Monitoring and Laboratory tests). Detailed instructions on the insertion and removal procedures of the implant are provided above [see Dosage and Administration (2.2)].
5.3 Psychiatric Events
Psychiatric events have been reported in patients taking GnRH agonists, including SUPPRELIN LA. Postmarketing reports with this class of drugs include symptoms of emotional lability, such as crying, irritability, impatience, anger, and aggression. Monitor for development or worsening of psychiatric symptoms during treatment with SUPPRELIN LA [see Adverse Reactions (6)].
5.4 Convulsions
Postmarketing reports of convulsions have been observed in patients receiving GnRH agonists, including SUPPRELIN LA. Reports with GnRH agonists have included patients with a history of seizures, epilepsy, cerebrovascular disorders, central nervous system anomalies or tumors, and patients on concomitant medications that have been associated with convulsions such as bupropion and SSRIs. Convulsions have also been reported in patients in the absence of any of the conditions mentioned above.
5.5 Pseudotumor Cerebri (Idiopathic Intracranial Hypertension)
Pseudotumor cerebri (idiopathic intracranial hypertension) have been reported in pediatric patients receiving GnRH agonists. Monitor patients for signs and symptoms of pseudotumor cerebri, including headache, papilledema, blurred vision, diplopia, loss of vision, pain behind the eye or pain with eye movement, tinnitus, dizziness, and nausea.
6. Adverse Reactions/Side Effects
The following serious adverse reactions are described here and elsewhere in the label:
- Initial Agonist Action [see Warnings and Precautions (5.1)]
- Implant Breakage [see Warnings and Precautions (5.2)]
- Psychiatric Events [see Warnings and Precautions (5.3)]
- Convulsions [see Warnings and Precautions (5.4)]
- Pseudotumor Cerebri (Idiopathic Intracranial Hypertension) [see Warnings and Precautions (5.5)]
6.1 Overall Adverse Reaction Profile
The most common adverse reactions with SUPPRELIN LA involved the implant site. Local reactions after implant insertion include bruising, pain, soreness, erythema and swelling.
During the early phase of therapy, gonadotropins and sex steroids rise above baseline because of the natural stimulatory effect of the drug. Therefore, an increase in clinical signs and symptoms may be observed [see Warnings and Precautions (5.1)].
6.2 Adverse Reactions in Clinical Trials
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of SUPPRELIN LA in children with CPP was evaluated in two single-arm clinical trials conducted in a total of 47 patients (44 females and 3 males) over a period of time ranging from 9 to 18 months. The most commonly reported adverse reaction was implant site reaction, which was reported by 24 of 47 (51.1%) patients. Implant site reaction includes discomfort, bruising, soreness, pain, tingling, itching, implant area protrusion and swelling. Two subjects experienced a serious adverse reaction: 1 subject who coincidentally had Stargardt’s Disease experienced amblyopia and 1 subject had a benign pituitary tumor (pituitary adenoma). One subject discontinued the study due to an adverse reaction of infection at the implant site. There were no clinically meaningful findings in standard clinical hematology and chemistry tests and/or in vital signs. The incidence of implantation adverse events reported by more than 2 patients are summarized in Table 1.
| Adverse Reactions | N=47 N (%) |
| Implant site reaction | 24 (51.1) |
| Keloid scar | 3 (6.4) |
| Scar | 3 (6.4) |
| Suture related complication | 3 (6.4) |
| Application site pain | 2 (4.3) |
| Post procedural pain | 2 (4.3) |
The following adverse reactions were reported as possibly related or related in 1 patient each: wound infection, breast tenderness, dysmenorrhea, epistaxis, erythema, feeling cold, gynecomastia, headache, menorrhagia, migraine, mood swings, pituitary tumor benign, pruritus, weight increased, disease progression and influenza-like illness. The adverse reaction metrorrhagia was reported as possibly related or related in 2 patients.
6.3 Post-marketing Experience
The following adverse reactions have been identified during post approval use of SUPPRELIN LA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
General Disorders and Administration Site Conditions: implant breakage
Psychiatric Disorders: Emotional lability, such as crying, irritability, impatience, anger, and aggression. Depression, including rare reports of suicidal ideation and attempt. Many, but not all, of these patients had a history of psychiatric illness or other comorbidities with an increased risk of depression.
Nervous System Disorders: seizures. Pseudotumor cerebri (idiopathic intracranial hypertension) have been observed with GnRH agonists.
Related/similar drugs
7. Drug Interactions
Overview: No formal drug-drug, drug-food, or drug-herb interaction studies were performed with SUPPRELIN LA.
Drug-Laboratory Interactions: Therapy with SUPPRELIN LA results in suppression of the pituitary-gonadal system. Results of diagnostic tests of pituitary gonadotropic and gonadal functions conducted during and after SUPPRELIN LA therapy may be affected. SUPPRELIN LA decreased mean serum insulin-like growth factor-1 (IGF-1) levels by approximately 11% in one study (Study 1). SUPPRELIN LA increased the serum concentration of dehydroepiandrosterone (DHEA) in 8 of 36 patients in another study (Study 2).
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
SUPPRELIN LA is contraindicated during pregnancy [see Contraindications (4)] since expected hormonal changes that occur with SUPPRELIN LA treatment increase the risk for pregnancy loss. The limited data with histrelin use in pregnant women are insufficient to determine a drug-associated risk for major birth defects or adverse developmental outcomes. Consistent with mechanism of action for SUPPRELIN LA [see Clinical Pharmacology (12.1)], animal reproduction studies showed an increase in fetal loss at clinically relevant exposures.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the US general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Animal Data
Histrelin acetate administered to pregnant rats during the period of organogenesis increased fetal mortality and post-implantation loss at doses of 1, 3, 5 or 15 mcg/kg/day, approximating clinical exposure based on body surface area. These dosages also reduced maternal body weight gain, stimulated ovarian follicular development, increased placental weight and caused abnormal morphology and an increase in fetal size. Histrelin acetate administered to pregnant rabbits during the period of organogenesis increased fetal mortality and abortion/early termination at the two highest doses and caused total litter loss at all doses of 20, 50 or 80 mcg/kg/day (approximately 3- to 12-times clinical exposures based on body surface area).
8.2 Lactation
Risk Summary
There are no data on the presence of SUPPRELIN LA in human or animal milk, the effects on the breastfed infant, or the effects on milk production. Absorption and systemic activity are not expected from potential exposure to the peptide, histrelin, in the breast milk. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for SUPPRELIN LA and any potential adverse effects on the breastfed child from SUPPRELIN LA or from the underlying maternal condition.
10. Overdosage
There have been no reports of overdose in SUPPRELIN LA clinical trials. High doses of histrelin acetate injection in animal studies were generally associated only with effects attributed to the expected pharmacology. The method of drug delivery makes accidental or intentional overdosage unlikely.
11. Supprelin LA Description
SUPPRELIN LA is a sterile, non-biodegradable, diffusion-controlled, hydrogel polymer reservoir containing histrelin acetate, a synthetic nonapeptide analog of the naturally occurring gonadotropin releasing hormone (GnRH) possessing a greater potency than the natural sequence hormone. SUPPRELIN LA is designed to deliver approximately 65 mcg histrelin acetate per day over 12 months.
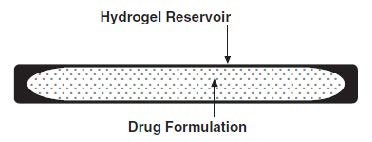
The SUPPRELIN LA implant looks like a small thin flexible tube and consists of a 50-mg histrelin acetate drug core inside a 3.5 cm by 3 mm, cylindrical, hydrogel polymer reservoir (Figure 1). The implant may appear partially to completely full with variation in color from off-white to light brown. The color may be uneven within the core.
Figure 1. SUPPRELIN LA Implant Diagram (not to scale)
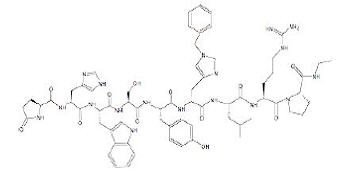
The chemical name of histrelin acetate is: L-Pyroglutamyl-L-histidyl-L-tryptophyl-L-seryl-L-tyrosyl-N-benzyl-D-histidyl-L-leucyl-L-arginyl-L-proline N-ethylamide, acetate salt.
The molecular formula for histrelin acetate is C66H86N18O12 x 2 CH3COOH and its molecular weight is 1443.70 (or 1323.52 as free base). Histrelin is also chemically described as 5-oxo-L-prolyl-L-histidyl-L-tryptophyl-L-seryl-L-tyrosyl-Nt-benzyl-D-histidyl-L-leucyl-L-arginyl-N-ethyl-L-prolinamide diacetate. The chemical structure of the free base (histrelin) is represented below in Figure 2.
Figure 2. Structure of Histrelin
The drug core also contains the inactive ingredient stearic acid NF. The hydrogel polymer reservoir is a hydrophilic cartridge composed of 2-hydroxyethyl methacrylate, 2-hydroxypropyl methacrylate, trimethylolpropane trimethacrylate, benzoin methyl ether, Perkadox-16, and Triton X-100. Each implant is packaged hydrated in a glass vial containing 2 mL of sterile 1.8% sodium chloride solution, so that it is primed for immediate release of the drug upon insertion.
A single use, sterile, Insertion Tool is provided along with the implant that can be used for the placement of the SUPPRELIN LA implant into the subcutaneous tissue of the inner aspect of the upper arm. The Insertion Tool is enclosed in a sterile bag and is provided separately from the implant in the Implantation Kit [see Recommended Procedure for Implant Insertion and Removal (2.2)].
12. Supprelin LA - Clinical Pharmacology
12.1 Mechanism of Action
SUPPRELIN LA is a GnRH agonist and an inhibitor of gonadotropin secretion when given continuously. It delivers approximately 65 mcg histrelin acetate per day. Both animal and human studies indicate that following an initial stimulatory phase, chronic, subcutaneous administration of histrelin acetate desensitizes responsiveness of the pituitary gonadotropin which, in turn causes a reduction in ovarian and testicular steroidogenesis.
In humans, administration of histrelin acetate results in an initial increase in circulating levels of LH and FSH, leading to a transient increase in concentration of gonadal steroids (testosterone and dihydrotestosterone in males, and estrone and estradiol in premenopausal females).
However, continuous administration of histrelin acetate causes a reversible down-regulation of the GnRH receptors in the pituitary gland and desensitization of the pituitary gonadotropes. These inhibitory effects result in decreased levels of LH and FSH.
12.2 Pharmacodynamics
Long-term treatment with histrelin acetate suppresses the LH response to GnRH causing LH levels to decrease to prepubertal levels within 1 month of treatment. As a result, serum concentrations of sex steroids (estrogen or testosterone) also decrease. Consequently, secondary sexual development ceases to progress in most patients. Additionally, linear growth velocity is slowed which improves the chance of attaining predicted adult height.
12.3 Pharmacokinetics
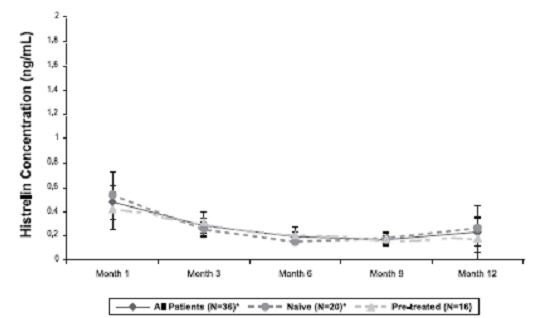
Pharmacokinetics of histrelin after implantation of SUPPRELIN LA was evaluated in a total of 47 children with CPP (11 subjects in Study 1 and 36 subjects in Study 2). Patients were examined at 4 weeks after implant insertion and a few times throughout the treatment period. Median serum histrelin concentrations remained above the limit of quantification for the treatment period. Histrelin acetate levels were sustained throughout the study period for most subjects (Figure 3). The median of maximum serum histrelin concentrations over the study period was 0.43 ng/mL, which is expected to maintain gonadotropins at prepubertal levels. There was no apparent pharmacokinetic difference between naïve subjects to a LHRH agonist treatment and subjects who had previous treatment with a LHRH agonist (Figure 3).
Figure 3. Mean and Standard Deviation of Serum Histrelin Concentrations (ng/mL) Results at Each Visit
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies were conducted in rats for 2 years at doses of 5, 25 or 150 mcg/kg/day (up to 11 times human exposures using body surface area comparisons, based on a 65 mcg/day dose in humans) and in mice for 18 months at doses of 20, 200, or 2000 mcg/kg/day (at less than therapeutic exposure to 70 times human exposure using body surface area comparisons, based on a 65 mcg/day dose in humans). As seen with other GnRH agonists, histrelin injection administration was associated with an increase in tumors of hormonally responsive tissues. There was a significant increase in pituitary adenomas in rats at mid and high doses (2-11 times human exposure based on body surface area comparisons with a 65 mcg/day human dose). There was an increase in pancreatic islet-cell adenomas in treated female rats and a non-dose-related increase in testicular Leydig-cell tumors (highest incidence in the low-dose group). In mice, there was significant increase in mammary-gland adenocarcinomas in all treated females. In addition, there were increases in stomach papillomas in male rats given high doses, and an increase in histiocytic sarcomas in female mice at the highest dose.
Mutagenicity studies have not been performed with histrelin acetate. Saline extracts of implants with and without histrelin acetate were negative in a battery of genotoxicity studies. Fertility studies have been conducted in rats and monkeys given subcutaneous daily doses of histrelin acetate up to 180 mcg/kg/day (up to 13 and 30 times human exposure, respectively using body surface area comparisons, based on a 65 mcg/day human dose) for 6 months and full reversibility of fertility suppression was demonstrated. The development and reproductive performance of offspring from parents treated with histrelin acetate has not been investigated.
14. Clinical Studies
The efficacy of SUPPRELIN LA in children with CPP has been evaluated in two single-arm, open label studies. Study 1 was conducted in 11 pretreated female patients, 3.7 to 11.0 years of age. Study 2 was conducted in 36 patients (33 females and 3 males), 4.5 to 11.6 years of age. Sixteen pretreated and 20 treatment-naïve patients were enrolled in Study 2. Baseline patient characteristics were typical of patients with CPP. Efficacy assessments were similar in both studies and included endpoints that measured the suppression of gonadotropins (luteinizing hormone and follicle stimulating hormone) and gonadal sex steroids (estrogen in girls and testosterone in boys, respectively) on treatment. Other assessments were clinical (evidence of stabilization or regression of signs of puberty) or gonadal steroid-dependent (bone age, linear growth). In Study 2, the primary measure of efficacy was LH suppression.
In Study 2, suppression of LH was induced in all treatment naïve subjects and maintained in all pretreated subjects at Month 1 after implantation and continued through Month 12 (suppression was defined as a peak LH < 4 mIU/mL following stimulation with the GnRH analog leuprolide acetate).
Secondary efficacy hormone assessments (FSH, estradiol and testosterone) and additional efficacy assessments (bone age advancement, linear growth, clinical progression of puberty) indicated stabilization of disease. Estradiol suppression was present in all 33 girls (100%) through Month 9 and 97% at Month 12. Testosterone suppression was maintained in the three pre-treated males participating in Study 2. The SUPPRELIN LA effect on efficacy endpoints in the Study 1 was consistent with that observed in Study 2.
16. How is Supprelin LA supplied
SUPPRELIN LA (NDC 67979-002-01) is supplied in a corrugated shipping carton that contains 2 inner cartons: a small one for the vial containing the SUPPRELIN LA implant, which is shipped with a cold pack inside a polystyrene cooler that must be refrigerated upon arrival, and a larger one comprising the Implantation Kit, which must not be refrigerated, for use during insertion or removal of SUPPRELIN LA.
The SUPPRELIN LA implant contains 50 mg of histrelin acetate. The SUPPRELIN LA implant carton contains a cold pack for refrigerated shipment and a small carton containing an amber plastic pouch. Inside the pouch is a glass vial with a Teflon-coated stopper and an aluminum seal, containing the implant in 2 mL of sterile 1.8% sodium chloride solution. (Note: The 3.5 mL vial is not completely filled with saline).
Upon receipt, refrigerate the small carton containing the amber plastic pouch and glass vial (with the implant inside) until the day of insertion. The implant vial should not be opened until just before the time of insertion.
SUPPRELIN LA is stable when stored refrigerated, in its sealed vial, pouch, and carton, at 2-8 °C (36-46 °F) until the expiration date provided. Excursion permitted to 25 °C (77 °F) for 7 days. Do not freeze. Protect from light.
17. Patient Counseling Information
Advise the patient to read the FDA-Approved patient labeling (Medication Guide).
Initial Agonistic Action
Patients should be advised that a transient worsening of symptoms of puberty or onset of new symptoms may occur initially. However, within 4 weeks of histrelin therapy, complete suppression of gonadal steroids occurs and manifestations of puberty decrease [see Warnings and Precautions (5.1)].
Post-insertion Care
Patients should be instructed to refrain from getting the inserted arm wet for 24 hours and from strenuous exertion of the inserted arm for 7 days after implant insertion to allow the incision to fully close. The adhesive elastic bandage can be removed at that time. The patient should not remove the surgical strips; rather, the strips should be allowed to fall off on their own after several days.
Psychiatric Adverse Events
Inform caregivers that symptoms of emotional lability, such as crying, irritability, impatience, anger, and aggression, have been observed in patients receiving GnRH agonists, including SUPPRELIN LA . Alert caregivers to the possibility of development or worsening of psychiatric symptoms, including depression, during treatment with SUPPRELIN LA [see Warnings and Precautions (5.3), Adverse Reactions (6.3)].
Convulsions
Inform caregivers that reports of convulsions have been observed in patients receiving GnRH agonists, including SUPPRELIN LA. Patients with a history of seizures, epilepsy, cerebrovascular disorders, central nervous system anomalies or tumors, and patients on concomitant medications that have been associated with convulsions may be at risk [see Warnings and Precautions (5.4)].
Pseudotumor Cerebri (Idiopathic Intracranial Hypertension)
Inform patients and caregivers that reports of pseudotumor cerebri (idiopathic intracranial hypertension) have been observed in pediatric patients receiving GnRH agonists. Advise patients and caregivers to monitor for headache, and vision issues such as blurred vision, double vision, loss of vision, pain behind the eye or pain with eye movement, ringing in the ears, dizziness, and nausea. Advise patients and caregivers to contact their healthcare provider if the patient develops any of these symptoms. [see Warnings and Precautions (5.5)].
Common Adverse Reactions
Patients should be advised to report to their physician any severe pain, redness, or swelling in and around the implant site. Infrequently, SUPPRELIN LA may be expelled from the body through the original incision site, rarely without the patient noticing. The patient should be instructed to monitor the incision site until it is healed. The patient should also return for routine checks of their condition and to ensure that SUPPRELIN LA is present and functioning in his/her body [see Adverse Reactions (6.1)].
For more information, call 1-800-462-3636 or visit www.supprelinla.com.
Distributed by:
Endo Pharmaceuticals Inc.
Malvern, PA 19355 USA
SUPPRELIN® is a registered trademark of Endo Pharmaceuticals Inc. or one of its affiliates.
©2019 Endo Pharmaceuticals Inc. All rights reserved.
Revised: 04/2022
Medication Guide
|
Medication Guide |
|
What is the most important information I should know about SUPPRELIN LA?
Call your child’s doctor right away if your child has any new or worsening mental symptoms or problems while taking SUPPRELIN LA.
Seizures have also happened in people who have not had any of these problems.
|
|
What is SUPPRELIN LA?
|
|
SUPPRELIN LA should not be taken if your child is:
|
|
Before your child receives SUPPRELIN LA, tell the doctor about all of your child’s medical conditions, including if they:
Tell your doctor about all the medicines your child takes, including prescription and over-the-counter medicines, vitamins, and herbal supplements. |
|
How will your child receive SUPPRELIN LA?
|
|
What are the possible side effects of SUPPRELIN LA? SUPPRELIN LA may cause serious side effects. See “What is the most important information I should know about SUPPRELIN LA?” The most common side effect of SUPPRELIN LA includes skin reactions at the place where the implant is inserted. These reactions may include pain, redness, bruising, soreness, and swelling in and around the implant site. Call your child’s doctor if your child has bleeding, redness, or severe pain where the implant was inserted. These are not all the possible side effects of SUPPRELIN LA. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|
What are the ingredients in SUPPRELIN LA? Active ingredient: histrelin acetate Inactive ingredients: stearic acid NF, hydrogel polymer reservoir composed of 2-hydroxyethyl methacrylate, 2-hydroxypropyl methacrylate, trimethylolpropane trimethacrylate, benzoin methyl ether, Perkadox-16, and Triton X-100 Distributed by: Endo Pharmaceuticals Inc., Malvern, PA 19355, www.endo.com or call 1-800-462-3636. |
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Rev: 04/2022
| SUPPRELIN
LA
histrelin acetate implant |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Endo Pharmaceuticals Inc. (178074951) |
Frequently asked questions
More about Supprelin LA (histrelin)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (2)
- Drug images
- Side effects
- Dosage information
- Patient tips
- During pregnancy
- FDA approval history
- Drug class: gonadotropin releasing hormones
- En español