Quillichew ER: Package Insert / Prescribing Info
Package insert / product label
Generic name: methylphenidate hydrochloride
Dosage form: tablet, chewable, extended release
Drug class: CNS stimulants
Medically reviewed by Drugs.com. Last updated on Nov 13, 2023.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Drug Interactions
- Use In Specific Populations
- Drug Abuse and Dependence
- Overdosage
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Storage and Handling
- Patient Counseling Information
- Medication Guide
Highlights of Prescribing Information
QUILLICHEW ER® (methylphenidate hydrochloride) extended-release chewable tablets, for oral use, CII
Initial U.S. Approval: 1955
WARNING: ABUSE, MISUSE, AND ADDICTION
See full prescribing information for complete boxed warning.
QUILLICHEW ER has a high potential for abuse and misuse, which can lead to the development of a substance use disorder, including addiction. Misuse and abuse of CNS stimulants, including QUILLICHEW ER, can result in overdose and death (5.1, 9.2, 10):
- Before prescribing QUILLICHEW ER, assess each patient’s risk for abuse, misuse, and addiction.
- Educate patients and their families about these risks, proper storage of the drug, and proper disposal of any unused drug.
- Throughout treatment, reassess each patient’s risk and frequently monitor for signs and symptoms of abuse, misuse, and addiction.
Recent Major Changes
Indications and Usage for Quillichew ER
QuilliChew ER is a central nervous system (CNS) stimulant indicated for the treatment of Attention Deficit Hyperactivity Disorder (ADHD). (1)
Quillichew ER Dosage and Administration
Dosage Forms and Strengths
Contraindications
Warnings and Precautions
- Risks to Patients with Serious Cardiac Disease: Avoid use in patients with known structural cardiac abnormalities, cardiomyopathy, serious cardiac arrhythmias, coronary artery disease, or other serious cardiac disease. (5.2)
- Increased Blood Pressure and Heart Rate: Monitor blood pressure and pulse. (5.3)
- Psychiatric Adverse Reactions: Prior to initiating QuilliChew ER, screen patients for risk factors for developing a manic episode. If new psychotic or manic symptoms occur, consider discontinuing QuilliChew ER. (5.4)
-
Priapism: If abnormally sustained or frequent and painful erections occur, patients should seek immediate medical attention. (5.5)
- Peripheral Vasculopathy, including Raynaud’s Phenomenon: Careful observation for digital changes is necessary during QuilliChew ER treatment. Further clinical evaluation (e.g., rheumatology referral) may be appropriate for patients who develop signs or symptoms of peripheral vasculopathy. (5.6)
- Long-Term Suppression of Growth in Pediatric Patients: Closely monitor growth (height and weight) in pediatric patients. Pediatric patients not growing or gaining height or weight as expected may need to have their treatment interrupted. (5.7)
- Risks in Phenylketonurics: QuilliChew ER extended-release chewable tablets contain phenylalanine, a component of aspartame. (5.8)
- Acute Angle Closure Glaucoma: QuilliChew ER -treated patients considered at risk for acute angle closure glaucoma (e.g., patients with significant hyperopia) should be evaluated by an ophthalmologist. (5.9)
- Increased Intraocular Pressure (IOP) and Glaucoma: Prescribe QuilliChew ER to patients with open-angle glaucoma or abnormally increased IOP only if the benefit of treatment is considered to outweigh the risk. Closely monitor patients with a history of increased IOP or open angle glaucoma. (5.10)
- Motor and Verbal Tics, and Worsening of Tourette’s Syndrome: Before initiating QuilliChew ER, assess the family history and clinically evaluate patients for tics or Tourette’s syndrome. Regularly monitor patients for the emergence or worsening of tics or Tourette’s syndrome. Discontinue treatment if clinically appropriate. (5.11)
Adverse Reactions/Side Effects
Based on accumulated data from other methylphenidate products, the most common (≥5% and twice the rate of placebo) adverse reactions are appetite decreased, insomnia, nausea, vomiting, dyspepsia, abdominal pain, weight decreased, anxiety, dizziness, irritability, affect lability, tachycardia, and blood pressure increased. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Tris Pharma, Inc. at (732) 940-0358 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Antihypertensive Drugs: Monitor blood pressure. Adjust dosage of antihypertensive drug as needed. (7)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 10/2023
Full Prescribing Information
WARNING: ABUSE, MISUSE, AND ADDICTION
QuilliChew ER has a high potential for abuse and misuse, which can lead to the development of a substance use disorder, including addiction. Misuse and abuse of CNS stimulants, including QuilliChew ER, can result in overdose and death [see Overdosage (10)], and this risk is increased with higher doses or unapproved methods of administration, such as snorting or injection.Before prescribing QuilliChew ER, assess each patient’s risk for abuse, misuse, and addiction. Educate patients and their families about these risks, proper storage of the drug, and proper disposal of any unused drug. Throughout QuilliChew ER treatment, reassess each patient’s risk of abuse, misuse, and addiction and frequently monitor for signs and symptoms of abuse, misuse, and addiction [see Warnings and Precautions (5.1) and Drug Abuse and Dependence (9.2)].
1. Indications and Usage for Quillichew ER
QuilliChew ER is indicated for the treatment of Attention Deficit Hyperactivity Disorder (ADHD) [see Clinical Studies (14)].
2. Quillichew ER Dosage and Administration
2.1 Pretreatment Screening
Prior to treating patients with QuilliChew ER, assess:
-
for the presence of cardiac disease (i.e., perform a careful history, family history of sudden death or ventricular arrhythmia, and physical exam) [see Warnings and Precautions (5.2) ].
- the family history and clinically evaluate patients for motor or verbal tics or Tourette’s syndrome before initiating QuilliChew ER [see Warnings and Precautions (5.10)].
2.2 Recommended Dosage
The recommended starting dosage of QuilliChew ER for patients 6 years and above is 20 mg once daily orally in the morning. The dose may be titrated up or down weekly in increments of 10 mg, 15 mg or 20 mg. The 10 mg and 15 mg doses can each be achieved by breaking in half the functionally scored 20 mg and 30 mg tablets, respectively. Daily doses above 60 mg have not been studied and are not recommended. As with any CNS stimulant, during titration of QuilliChew ER, the prescribed dose should be adjusted, if necessary, until a well-tolerated, therapeutic dose is achieved.
2.3 Administration Instructions
QuilliChew ER should be orally administered once daily in the morning with or without food [see Clinical Pharmacology (12.3)].
2.4 Switching from other Methylphenidate Products
If switching from other methylphenidate products, discontinue that treatment, and titrate with QuilliChew ER using the above titration schedule.
Do not substitute for other methylphenidate products on a milligram-per-milligram basis, because of different methylphenidate base compositions and differing pharmacokinetic profiles [see Description (11), Clinical Pharmacology (12.3)].
3. Dosage Forms and Strengths
Extended-release chewable tablets:
20 mg equivalent of methylphenidate HCl available as a speckled, off-white, capsule-shaped coated tablet, debossed with "NP 12" on one side and functionally scored on the other side.
30 mg equivalent of methylphenidate HCl available as a speckled, light pink color, capsule-shaped coated tablet, debossed with "NP 13" on one side and functionally scored on the other side.
40 mg equivalent of methylphenidate HCl available as a speckled, dark pink to peach color, capsule-shaped coated tablet, debossed with "NP 14" on one side and plain (not scored) on the other side.
4. Contraindications
4.1 Hypersensitivity to Methylphenidate or other Components of QuilliChew ER
QuilliChew ER is contraindicated in patients known to be hypersensitive to methylphenidate, or other components of QuilliChew ER. Hypersensitivity reactions such as angioedema and anaphylactic reactions have been reported in patients treated with other methylphenidate products [see Adverse Reactions (6.2)].
4.2 Monoamine Oxidase Inhibitors
QuilliChew ER is contraindicated during concomitant treatment with monoamine oxidase inhibitors (MAOIs), and also within 14 days following discontinuation of treatment with a monoamine oxidase inhibitor (MAOI), because of the risk of hypertensive crisis [see Drug Interactions (7.1)].
5. Warnings and Precautions
5.1 Abuse, Misuse, and Addiction
QuilliChew ER has a high potential for abuse and misuse. The use of QuilliChew ER exposes individuals to the risks of abuse and misuse, which can lead to the development of a substance use disorder, including addiction. QuilliChew ER can be diverted for non-medical use into illicit channels or distribution [see Drug Abuse and Dependence (9.2)]. Misuse and abuse of CNS stimulants, including QuilliChew ER, can result in overdose and death [see Overdosage (10)], and this risk is increased with higher doses or unapproved methods of administration, such as snorting or injection.
Before prescribing QuilliChew ER, assess each patient’s risk for abuse, misuse, and addiction.Educate patients and their families about these risks and proper disposal of any unused drug. Advise patients to store QuilliChew ER in a safe place, preferably locked, and instruct patients to not give QuilliChew ER to anyone else. Throughout QuilliChew ER treatment, reassess each patient’s risk of abuse, misuse, and addiction and frequently monitor for signs and symptoms of abuse, misuse, and addiction.
5.2 Risks to Patients with Serious Cardiac Disease
Sudden death has occurred in patients with structural cardiac abnormalities or other serious cardiac disease who were treated with CNS stimulants at the recommended ADHD dosage.
Avoid QuilliChew ER use in patients with known structural cardiac abnormalities, cardiomyopathy, serious cardiac arrhythmias, coronary artery disease, or other serious cardiac disease.
5.3 Increased Blood Pressure and Heart Rate
CNS stimulants cause an increase in blood pressure (mean increase approximately 2 to 4 mmHg) and heart rate (mean increase approximately 3 to 6 bpm). Some patients may have larger increases.
Monitor all QuilliChew ER-treated patients for hypertension and tachycardia.
5.4 Psychiatric Adverse Reactions
Exacerbation of Pre-existing Psychosis
CNS stimulants may exacerbate symptoms of behavior disturbance and thought disorder in patients with a pre-existing psychotic disorder.
Induction of a Manic Episode in Patients with Bipolar Disorder
CNS stimulants may induce a manic or mixed episode in patients. Prior to initiating QuilliChew ER treatment, screen patients for risk factors for developing a manic episode (e.g., comorbid or history of depressive symptoms or a family history of suicide, bipolar disorder, or depression).
New Psychotic or Manic Symptoms
CNS stimulants, at the recommended dosage, may cause psychotic or manic symptoms (e.g., hallucinations, delusional thinking, or mania) in patients without a prior history of psychotic illness or mania. In a pooled analysis of multiple short-term, placebo-controlled studies of CNS stimulants, psychotic or manic symptoms occurred in approximately 0.1% of CNS stimulant-treated patients, compared to 0% of placebo-treated patients. If such symptoms occur, consider discontinuing QuilliChew ER.
5.5 Priapism
Prolonged and painful erections, sometimes requiring surgical intervention, have been reported with methylphenidate use in both adult and pediatric male patients. Although priapism was not reported with methylphenidate initiation, it developed after some time on methylphenidate, often subsequent to an increase in dosage. Priapism also occurred during a period of methylphenidate withdrawal (drug holidays or during discontinuation).
QuilliChew ER treated patients who develop abnormally sustained or frequent and painful erections should seek immediate medical attention.
5.6 Peripheral Vasculopathy, including Raynaud’s Phenomenon
CNS stimulants, including QuilliChew ER, used to treat ADHD are associated with peripheral vasculopathy, including Raynaud's phenomenon. Signs and symptoms are usually intermittent and mild; however, sequelae have included digital ulceration and/or soft tissue breakdown. Effects of peripheral vasculopathy, including Raynaud's phenomenon, were observed in post-marketing reports and at the therapeutic dosages of CNS stimulants in all age groups throughout the course of treatment. Signs and symptoms generally improved after dosage reduction or discontinuation of the CNS stimulant.
Careful observation of digital changes is necessary during QuilliChew ER treatment. Further clinical evaluation (e.g., rheumatology referral) may be appropriate for QuilliChew ER-treated patients who develop signs or symptoms of peripheral vasculopathy.
5.7 Long-Term Suppression of Growth in Pediatric Patients
CNS stimulants have been associated with weight loss and slowing of growth rate in pediatric patients.
Careful follow-up of weight and height in pediatric patients ages 7 to 10 years who were randomized to either methylphenidate or nonmedication treatment groups over 14 months, as well as in naturalistic subgroups of newly methylphenidate-treated and nonmedication-treated pediatric patients over 36 months (to the ages of 10 to 13 years), suggests that pediatric patients who received methylphenidate for 7 days per week throughout the year had a temporary slowing in growth rate (on average, a total of about 2 cm less growth in height and 2.7 kg less growth in weight over 3 years), without evidence of growth rebound during this development period.
Closely monitor growth (weight and height) in QuilliChew ER-treated pediatric patients. Pediatric patients who are not growing or gaining height or weight as expected may need to have their treatment interrupted.
5.8 Risks in Patients with Phenylketonuria
Phenylalanine can be harmful to patients with phenylketonuria (PKU). QuilliChew ER extended-release chewable tablets contain phenylalanine, a component of aspartame. Each 20 mg, 30 mg, and 40 mg extended-release chewable tablet contains 3 mg, 4.5 mg, and 6 mg phenylalanine, respectively. Before prescribing QuilliChew ER in patients with PKU, consider the combined daily amount of phenylalanine from all sources, including QuilliChew ER.
5.9 Acute Angle Closure Glaucoma
There have been reports of angle closure glaucoma associated with methylphenidate treatment.
Although the mechanism is not clear, QuilliChew ER -treated patients considered at risk for acute angle closure glaucoma (e.g., patients with significant hyperopia) should be evaluated by an ophthalmologist.
5.10 Increased Intraocular Pressure and Glaucoma
There have been reports of an elevation of intraocular pressure (IOP) associated with methylphenidate treatment [see Adverse Reactions (6.2)].
Prescribe QuilliChew ER to patients with open-angle glaucoma or abnormally increased IOP only if the benefit of treatment is considered to outweigh the risk. Closely monitor QuilliChew ER -treated patients with a history of abnormally increased IOP or open angle glaucoma.
5.11 Motor and Verbal Tics, and Worsening of Tourette’s Syndrome
CNS stimulants, including methylphenidate, have been associated with the onset or exacerbation of motor and verbal tics. Worsening of Tourette’s syndrome has also been reported [see Adverse Reactions (6.2)].
Before initiating QuilliChew ER, assess the family history and clinically evaluate patients for tics or Tourette’s syndrome. Regularly monitor QuilliChew ER-treated patients for the emergence or worsening of tics or Tourette’s syndrome, and discontinue treatment if clinically appropriate.
6. Adverse Reactions/Side Effects
The following are discussed in more detail in other sections of the labeling:
• Known hypersensitivity to methylphenidate products or other ingredients of QuilliChew ER [see Contraindications (4.1)]
• Hypertensive Crisis When Used Concomitantly with Monoamine Oxidase Inhibitors [see Contraindications (4.2), Drug Interactions (7.1)]
• Abuse, Misuse, and Addiction [see Boxed Warning, Warnings and Precautions (5.1), Drug Abuse and Dependence (9.2, 9.3)]
• Risks to Patients with Serious Cardiac Disease [see Warnings and Precautions (5.2)]
• Increased Blood Pressure and Heart Rate [see Warnings and Precautions (5.3)]
• Psychiatric Adverse Reactions [see Warnings and Precautions (5.4)]
• Priapism [see Warnings and Precautions (5.5)]
• Peripheral Vasculopathy, including Raynaud’s phenomenon [see Warnings and Precautions (5.6)]
• Long-Term Suppression of Growth in Pediatric Patients [see Warnings and Precautions (5.7)]
• Risks in Phenylketonuria [see Warnings and Precautions (5.8)]
• Acute Angle Closure Glaucoma [see Warnings and Precautions (5.9)]
• Increased Intraocular Pressure and Glaucoma [see Warnings and Precautions (5.10)]
• Motor and Verbal Tics, and Worsening of Tourette’s Syndrome [see Warnings and Precautions (5.11)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Adverse Reactions in Studies with Other Methylphenidate Products in Children, Adolescents, and Adults with ADHD
Commonly reported (≥2% of the methylphenidate group and at least twice the rate of the placebo group) adverse reactions from placebo-controlled trials of methylphenidate products include: appetite decreased, weight decreased, nausea, abdominal pain, dyspepsia, dry mouth, vomiting, insomnia, anxiety, nervousness, restlessness, affect lability, agitation, irritability, dizziness, vertigo, tremor, blurred vision, blood pressure increased, heart rate increased, tachycardia, palpitations, hyperhidrosis, and pyrexia.
Adverse Reactions in Studies with QuilliChew ER in Children with ADHD
There is limited experience with QuilliChew ER in controlled trials. The safety data in this section is based on data from a laboratory classroom study conducted in 90 pediatric subjects (ages 6 to 12 years) with ADHD. The study consisted of a 6-week dose optimization period, followed by a randomized, double-blind, parallel group treatment period with the individually optimized dose of QuilliChew ER or placebo.
The most common (≥2% in the QuilliChew ER group and greater than placebo) adverse reactions reported in the double-blind, randomized, placebo-controlled phase in patients optimized to doses of QuilliChew ER 20 to 60 mg/day are described in Table 1.
| Adverse reaction | QuilliChew ER
N= 42 n (%) | Placebo
N= 44 n (%) |
| Decreased appetite | 1 (2.4) | 0 (0) |
| Aggression | 1 (2.4) | 0 (0) |
| Emotional poverty | 1 (2.4) | 0 (0) |
| Nausea | 1 (2.4) | 0 (0) |
| Headache | 1 (2.4) | 0 (0) |
| Weight decreased | 1 (2.4) | 0 (0) |
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of methylphenidate products. Because these reactions are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These adverse reactions are as follows:
Blood and Lymphatic System Disorders: Pancytopenia, Thrombocytopenia, Thrombocytopenic purpura
Cardiac Disorders: Angina pectoris, Bradycardia, Extrasystole, Supraventricular tachycardia, Ventricular extrasystole
Eye Disorders: Diplopia, Increased intraocular pressure, Mydriasis, Visual impairment
General Disorders: Chest pain, Chest discomfort, Hyperpyrexia
Hepatobiliary Disorders: Severe hepatocellular injury
Immune System Disorders: Hypersensitivity reactions such as Angioedema, Anaphylactic reactions, Auricular swelling, Bullous conditions, Exfoliative conditions, Urticarias, Pruritus NEC, Rashes, Eruptions, and Exanthemas NEC
Investigations: Alkaline phosphatase increased, Bilirubin increased, Hepatic enzyme increased, Platelet count decreased, White blood cell count abnormal
Musculoskeletal, Connective Tissue and Bone Disorders: Arthralgia, Myalgia, Muscle twitching, Rhabdomyolysis
Nervous System Disorders: Convulsion, Grand mal convulsion, Dyskinesia, Serotonin syndrome in combination with serotonergic drugs, Motor and Verbal Tics
Psychiatric Disorders: Disorientation, Hallucination, Hallucination auditory, Hallucination visual, Libido changes, Mania
Urogenital System: Priapism
Skin and Subcutaneous Tissue Disorders: Alopecia, Erythema
Vascular Disorders: Raynaud’s phenomenon
Related/similar drugs
7. Drug Interactions
7.1 Clinically Important Drug Interactions
MAOI Inhibitors
Do not administer QuilliChew ER concomitantly with monoamine oxidase inhibitors (MAOIs) or within 14 days after discontinuing MAOI treatment. Concomitant use of MAOIs and CNS stimulants can cause hypertensive crisis. Potential outcomes include death, stroke, myocardial infarction, aortic dissection, ophthalmological complications, eclampsia, pulmonary edema, and renal failure.
Antihypertensive Drugs
QuilliChew ER may decrease the effectiveness of drugs used to treat hypertension. Monitor blood pressure and adjust the dosage of the hypertensive drug as needed [see Warnings and Precautions (5.3)].
Halogenated Anesthetics
Concomitant use of halogenated anesthetics and QuilliChew ER may increase the risk of sudden blood pressure and heart rate increase during surgery. Avoid use of QuilliChew ER in patients being treated with anesthetics on the day of surgery.
Risperidone
Combined use of methylphenidate with risperidone when there is a change, whether an increase or decrease, in dosage of either or both medications, may increase the risk of extrapyramidal symptoms (EPS). Monitor for signs of EPS.
8. Use In Specific Populations
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to ADHD medications during pregnancy. Healthcare providers are encouraged to register patients by calling the National Pregnancy Registry for Psychostimulants at 1-866-961-2388 or visiting online at https://womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry/othermedications/.
Risk Summary
There are limited published studies and small case series that report on the use of methylphenidate in pregnant women; however, the data are insufficient to inform any drug-associated risks. There are clinical considerations [see Clinical Considerations]. No teratogenic effects were observed in embryo-fetal development studies with oral administration of methylphenidate to pregnant rats and rabbits during organogenesis at doses 2 and 11 times, respectively, the maximum recommended human dose (MRHD). However, spina bifida was observed in rabbits at a dose 40 times the MRHD [see Data].
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Fetal/Neonatal adverse reactions
CNS stimulant medications, such as QuilliChew ER, can cause vasoconstriction and thereby decrease placental perfusion. No fetal and/or neonatal adverse reactions have been reported with the use of therapeutic doses of methylphenidate during pregnancy; however, premature delivery and low birth weight infants have been reported in amphetamine-dependent mothers.
Data
Animal Data
In studies conducted in rats and rabbits, methylphenidate was administered orally at doses of up to 75 and 200 mg/kg/day, respectively, during the period of organogenesis. Teratogenic effects (increased incidence of fetal spina bifida) were observed in rabbits at the highest dose, which is approximately 40 times the maximum recommended human dose (MRHD) on a mg/m2 basis. The no effect level for embryo-fetal development in rabbits was 60 mg/kg/day (11 times the MRHD on a mg/m2 basis). There was no evidence of specific teratogenic activity in rats, although increased incidences of fetal skeletal variations were seen at the highest dose level (7 times the MRHD on a mg/m2 basis), which was also maternally toxic. The no effect level for embryo-fetal development in rats was 25 mg/kg/day (2 times the MRHD on a mg/m2 basis).
8.2 Lactation
Risk Summary
Limited published literature reports that methylphenidate is present in human milk, which resulted in infant doses of 0.16% to 0.7% of the maternal weight-adjusted dosage and a milk/plasma ratio ranging between 1.1 and 2.7. There are no reports of adverse effects on the breastfed infant and no effects on milk production. Long-term neurodevelopmental effects on infants from CNS stimulant exposure are unknown. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for QuilliChew ER and any potential adverse effects on the breastfed infant from QuilliChew ER or from the underlying maternal condition.
Clinical Considerations
Monitor breastfeeding infants for adverse reactions, such as agitation, insomnia, anorexia, and reduced weight gain.
8.4 Pediatric Use
The safety and effectiveness of QuilliChew ER have been established in pediatric patients ages 6 to 17 years. Use of QuilliChew ER in these age groups is based on one adequate and well-controlled clinical study in pediatric patients 6 to 12 years old, pharmacokinetic data in adolescents and adults, and safety information from other methylphenidate-containing products. The safety and effectiveness of QuilliChew ER in pediatric patients less than 6 years have not been established.
The long-term efficacy of methylphenidate in pediatric patients has not been established.
Long Term Suppression of Growth
Growth should be monitored during treatment with CNS stimulants, including QuilliChew ER. Children who are not growing or gaining weight as expected may need to have their treatment interrupted [see Warnings and Precautions (5.7)].
Juvenile Animal Data
Rats treated with methylphenidate early in the postnatal period through sexual maturation demonstrated a decrease in spontaneous locomotor activity in adulthood. A deficit in acquisition of a specific learning task was observed in females only. The doses at which these findings were observed are at least 6 times the maximum recommended human dose (MRHD) on a mg/m2 basis.
In the study conducted in young rats, methylphenidate was administered orally at doses of up to 100 mg/kg/day for 9 weeks, starting early in the postnatal period (postnatal day 7) and continuing through sexual maturity (postnatal week 10). When these animals were tested as adults (postnatal weeks 13 to 14), decreased spontaneous locomotor activity was observed in males and females previously treated with 50 mg/kg/day (approximately 6 times the maximum recommended human dose [MRHD] on a mg/m2 basis) or greater, and a deficit in the acquisition of a specific learning task was observed in females exposed to the highest dose (12 times the MRHD on a mg/m2 basis). The no effect level for juvenile neurobehavioral development in rats was 5 mg/kg/day (half the MRHD on a mg/m2 basis). The clinical significance of the long-term behavioral effects observed in rats is unknown.
9. Drug Abuse and Dependence
9.1 Controlled Substance
QuilliChew ER contains methylphenidate, a Schedule II controlled substance.
9.2 Abuse
QuilliChew ER has a high potential for abuse and misuse which can lead to the development of a substance use disorder, including addiction [see Warnings and Precautions (5.1)].
QuilliChew ER can be diverted for non-medical use into illicit channels or distribution.
Abuse is the intentional non-therapeutic use of a drug, even once, to achieve a desired psychological or physiological effect. Misuse is the intentional use, for therapeutic purposes, of a drug by an individual in a way other than prescribed by a health care provider or for whom it was not prescribed. Drug addiction is a cluster of behavioral, cognitive, and physiological phenomena that may include a strong desire to take the drug, difficulties in controlling drug use (e.g., continuing drug use despite harmful consequences, giving a higher priority to drug use than other activities and obligations), and possible tolerance or physical dependence.
Misuse and abuse of methylphenidate may cause increased heart rate, respiratory rate, or blood pressure; sweating; dilated pupils; hyperactivity; restlessness; insomnia; decreased appetite; loss of coordination; tremors; flushed skin; vomiting; and/or abdominal pain. Anxiety, psychosis, hostility, aggression, and suicidal or homicidal ideation have also been observed with CNS stimulants abuse and/or misuse. Misuse and abuse of CNS stimulants, including QuilliChew ER, can result in overdose and death [see Overdosage (10)], and this risk is increased with higher doses or unapproved methods of administration, such as snorting or injection.
9.3 Dependence
Physical Dependence
QuilliChew ER may produce physical dependence. Physical dependence is a state that develops as a result of physiological adaptation in response to repeated drug use, manifested by withdrawal signs and symptoms after abrupt discontinuation or a significant dose reduction of a drug.
Withdrawal signs and symptoms after abrupt discontinuation or dose reduction following prolonged use of CNS stimulants including QuilliChew ER include dysphoric mood; depression; fatigue; vivid, unpleasant dreams; insomnia or hypersomnia; increased appetite; and psychomotor retardation or agitation.
Tolerance
QuilliChew ER may produce tolerance. Tolerance is a physiological state characterized by a reduced response to a drug after repeated administration (i.e., a higher dose of a drug is required to produce the same effect that was once obtained at a lower dose).
10. Overdosage
Clinical Effects of Overdose
Overdose of CNS stimulants is characterized by the following sympathomimetic effects:
- Cardiovascular effects including tachyarrhythmias, and hypertension or hypotension. Vasospasm, myocardial infarction, or aortic dissection may precipitate sudden cardiac death. Takotsubo cardiomyopathy may develop.
- CNS effects including psychomotor agitation, confusion, and hallucinations. Serotonin syndrome, seizures, cerebral vascular accidents, and coma may occur.
- Life-threatening hyperthermia (temperatures greater than 104°F) and rhabdomyolysis may develop.
Overdose Management
Consider the possibility of multiple drug ingestion. The pharmacokinetic profile of QuilliChew ER should be considered when treating patients with overdose. Because methylphenidate has a large volume of distribution and is rapidly metabolized, dialysis is not useful. Consider contacting the Poison Help line (1-800-222-1222) or a medical toxicologist for additional overdose management recommendations.
11. Quillichew ER Description
QuilliChew ER (methylphenidate hydrochloride extended-release chewable tablets) is available in three dosage strengths - 20 mg, 30 mg and 40 mg. The dosage strengths are expressed in terms of methylphenidate hydrochloride equivalents; however only 15% of methylphenidate is present as methylphenidate hydrochloride salt. The remaining 85% is present as methylphenidate ionically-bound to the sulfonate groups of sodium polystyrene sulfonate particles. QuilliChew ER contains approximately 30% immediate-release and 70% extended-release methylphenidate.
The QuilliChew ER extended-release chewable tablets are cherry flavored.
Methylphenidate HCl is a central nervous system (CNS) stimulant. The chemical name is methyl α- phenyl-2-piperidineacetate hydrochloride, and its structural formula is shown in Figure 1.
Figure 1: Methylphenidate HCl Structure
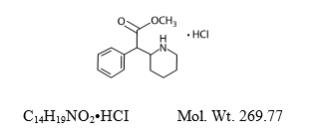
Methylphenidate HCl is a white, odorless crystalline powder. Its solutions are acid to litmus. It is freely soluble in water and in methanol, soluble in alcohol, and slightly soluble in chloroform and in acetone.
QuilliChew ER also contains the following inactive ingredients: aspartame [see Warnings and Precautions (5.8)], cherry flavor, citric acid, crospovidone, D&C red #30 (for 30 mg strength), D&C red #7 (for 40 mg strength), guar gum, magnesium stearate, mannitol, microcrystalline cellulose, polyvinyl acetate, polyvinyl alcohol, povidone, silicon dioxide, sodium polystyrene sulfonate, talc, triacetin, xanthan gum.
12. Quillichew ER - Clinical Pharmacology
12.2 Pharmacodynamics
Methylphenidate is a racemic mixture comprised of the d- and l-isomers. The d-isomer is more pharmacologically active than the l-isomer. The mode of therapeutic action in ADHD is not known. Methylphenidate blocks the reuptake of norepinephrine and dopamine into the presynaptic neuron and increases the release of these monoamines into the extraneuronal space.
12.3 Pharmacokinetics
Absorption
Following a single oral dose of 40 mg QuilliChew ER under fasting conditions, plasma methylphenidate reached maximal concentration (Cmax ) at a median time of 5 hours after dosing. Compared to an immediate-release formulation of methylphenidate chewable tablet (40 mg in 2 equal doses of 20 mg, 6 hours apart), methylphenidate mean peak concentration and exposure (AUCinf) was about 20% and 11% lower, respectively, after single dose administration of 40 mg QuilliChew ER (Figure 2).
Figure 2: Mean Methylphenidate Plasma Concentration-Time Profiles After Administration of 40 mg QuilliChew ER or Methylphenidate Immediate-Release Chewable Tablets (IRCT, 2 Equal Doses of 20 mg, 6 Hours Apart) Under Fasted Conditions in Healthy Volunteers
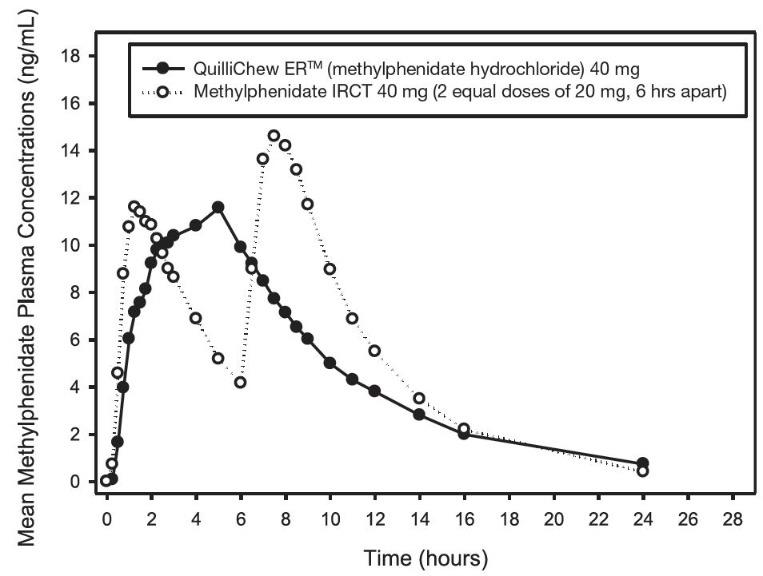
Food Effect
High-fat meal had no effect on the time to peak concentration, and increased Cmax and systemic exposure (AUCinf) of methylphenidate by about 20% and 4%, respectively, after a single dose administration of 40 mg QuilliChew ER.
Elimination
Plasma methylphenidate concentrations decline monophasically following oral administration of QuilliChew ER. The mean plasma terminal elimination half-life of methylphenidate was about 5.2 hours in healthy volunteers following a single 40 mg dose administration.
Metabolism
In humans, methylphenidate is metabolized primarily via de-esterification to alpha-phenyl-piperidine acetic acid (PPAA). The metabolite has little or no pharmacologic activity.
Excretion
After oral dosing of radiolabeled methylphenidate in humans, about 90% of the radioactivity was recovered in urine. The main urinary metabolite was PPAA, accounting for approximately 80% of the dose.
Alcohol Effect
At 40% alcohol concentration, there was about 90% release methylphenidate from QuilliChew ER 40 mg tablet within half an hour. The results with the 40 mg chewable tablet strength are considered representative of the other available tablet strengths.
Specific Populations
Sex
There is insufficient experience with the use of QuilliChew ER to detect gender variations in pharmacokinetics.
Race
There is insufficient experience with the use of QuilliChew ER to detect ethnic variations in pharmacokinetics.
Age
There are no specific pediatric pharmacokinetic studies for QuilliChew ER. However, the pharmacokinetics of methylphenidate in pediatric patients 6 to 17 years old are not expected to be significantly different from adults following QuilliChew ER administration.
Renal Impairment
There is no experience with the use of QuilliChew ER in patients with renal insufficiency. After oral administration of radiolabeled methylphenidate in humans, methylphenidate was extensively metabolized and approximately 80% of the radioactivity was excreted in the urine in the form of PPAA. Since renal clearance is not an important route of methylphenidate clearance, renal insufficiency is expected to have little effect on the pharmacokinetics of QuilliChew ER.
Hepatic Impairment
There is no experience with the use of QuilliChew ER in patients with hepatic insufficiency.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
In a lifetime carcinogenicity study carried out in B6C3F1 mice, methylphenidate caused an increase in hepatocellular adenomas and, in males only, an increase in hepatoblastomas, at a daily dose of approximately 60 mg/kg/day. This dose is approximately 4 times the maximum recommended human dose on a mg/m2 basis. Hepatoblastoma is a relatively rare rodent malignant tumor type. There was no increase in total malignant hepatic tumors. The mouse strain used is sensitive to the development of hepatic tumors, and the significance of these results to humans is unknown.
Methylphenidate did not cause any increase in tumors in a lifetime carcinogenicity study carried out in F344 rats; the highest dose used was approximately 45 mg/kg/day, which is approximately 5 times the maximum recommended human dose on a mg/m2 basis.
Mutagenesis
Methylphenidate was not mutagenic in the in vitro Ames reverse mutation assay or in the in vitro mouse lymphoma cell forward mutation assay. Sister chromatid exchanges and chromosome aberrations were increased, indicative of a weak clastogenic response, in an in vitro assay in cultured Chinese Hamster Ovary (CHO) cells. Methylphenidate was negative in an in vivo mouse bone marrow micronucleus assay.
Impairment of Fertility
Methylphenidate did not impair fertility in male or female mice that were fed diets containing the drug in an 18-week Continuous Breeding study. The study was conducted at doses of up to 160 mg/kg/day, approximately 8-fold the maximum recommended human dose on a mg/m2 basis.
14. Clinical Studies
The efficacy of QuilliChew ER was evaluated in a laboratory classroom study conducted in 90 pediatric subjects (ages 6 to 12 years) with ADHD. Patients in the trial met DSM-IV criteria for ADHD. The study began with a 6-week open-label dose optimization period with an initial QuilliChew ER dose of 20 mg. Patients were instructed to chew each dose once daily in the morning. The dose could be titrated weekly in increments of 10 to 20 mg until a therapeutic dose or the maximum dose of 60 mg/day was reached.
Eighty-six of the 90 enrolled subjects then entered a 1-week randomized, double-blind, parallel group treatment period with the individually optimized dose of QuilliChew ER or placebo. The intent-to-treat (ITT) population consisted of 85 randomized subjects who received at least 1 dose of double-blind study drug and had at least 1 post-Baseline assessment of the primary efficacy variable. At the end of the double-blind treatment period, the laboratory classroom raters and teachers evaluated the attention and behavior of the subjects, throughout the day using the Swanson, Kotkin, Agler, M-Flynn, and Pelham (SKAMP) rating scale. The SKAMP rating scale is a validated 13-item teacher-rated scale that assesses manifestations of ADHD in a classroom setting.
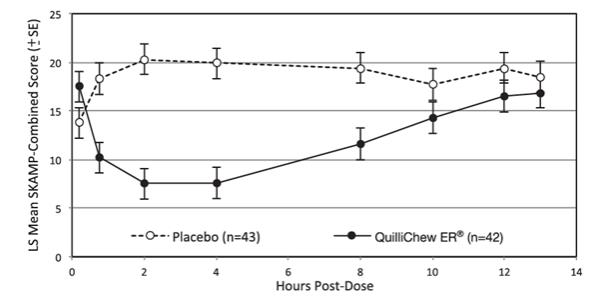
The SKAMP-Combined score, measured at 0.75, 2, 4, 8, 10, 12, and 13 hours post-dose during the laboratory classroom day at the end of the double-blind treatment period, was used to assess the primary and the key secondary efficacy parameters. The primary efficacy endpoint was the average of treatment effects across all the time points as specified above during the classroom day. The key secondary efficacy parameters were onset and duration of clinical effect. QuilliChew ER was statistically significantly superior to placebo with respect to the primary endpoint (Table 2). QuilliChew ER also showed improvement over placebo at 0.75, 2, 4, and 8 hours post-dosing. Efficacy results at each time point are summarized in Figure 3.
| Study Number | Treatment Group | Primary Efficacy measure: Average of Treatment Effect Across All Time Points Based on SKAMP-Combined Score | ||
| Mean Pre-Dose Score on Classroom Day (SD) | LS Mean (SE) for the Classroom day | Placebo-subtracted Differencea (95% CI) |
||
| Study 1 | Quillichew ER (N=42) | 17.5 (11.6) | 12.1 (1.4) | -7.0 (-10.9, -3.1) |
| Placebo (N-43) | 13.8 (10.0) | 19.1 (1.4) | ||
N: number of patients; SD: standard deviation; SE: standard error; LS Mean: least-squares
mean; CI: confidence interval.
aLeast-Squares Mean Difference (drug minus placebo).
Figure 3: SKAMP-Combined Scores Over Time (LS Mean ±SE) by Treatment Group (ITT Population)
ITT: intent-to-treat
LS means from post-dose time-points were obtained from a repeated measures mixed model with terms for center, hour, treatment and treatment by hour interaction. For the pre-dose time-point, arithmetic means and standard errors are displayed.
16. How is Quillichew ER supplied
16.1 How Supplied
QuilliChew ER is supplied as extended-release chewable tablets in 20 mg, 30 mg and 40 mg strengths.
The 20 mg strength extended-release chewable tablet is available as a speckled, off-white, capsule-shaped coated tablet, debossed with "NP 12" on one side and functionally scored on the other side.
The 30 mg strength extended-release chewable tablet is available as a speckled, light pink color, capsule-shaped coated tablet, debossed with "NP 13" on one side and functionally scored on the other side.
The 40 mg strength extended-release chewable tablet is available as a speckled, dark pink to peach color, capsule-shaped coated tablet, debossed with "NP 14" on one side and plain on the other side (not scored).
The product is supplied in bottles of 100.
| QuilliChew ER extended-release chewable tablets | |||
| Package Configuration | Tablet Strength (mg) | NDC | |
| Bottles of 100 | 20 mg | NDC-24478-074-01 | NP 12 |
| Bottles of 100 | 30 mg | NDC-24478-075-01 | NP 13 |
| Bottles of 100 | 40 mg | NDC-24478-076-01 | NP 14 |
17. Patient Counseling Information
Advise patients to read the FDA-approved patient labeling (Medication Guide).
Abuse, Misuse, and Addiction
Educate patients and their families about the risks of abuse, misuse, and addiction of QuilliChew ER, which can lead to overdose and death, and proper disposal of any unused drug [see Warnings and Precautions (5.1), Drug Abuse and Dependence (9.2), Overdosage (10)].
Advise patients to store QuilliChew ER in a safe place, preferably locked, and instruct patients to not give QuilliChew ER to anyone else.
Dosage and Administration Instructions
Advise patients that QuilliChew ER should be taken by mouth once daily in the morning with or without food.
Risks to Patients with Serious Cardiac Disease
Advise patients that there are potential risks to patients with serious cardiac disease, including sudden death, with QuilliChew ER use. Instruct patients to contact a health care provider immediately if they develop symptoms such as exertional chest pain, unexplained syncope, or other symptoms suggestive of cardiac disease [see Warnings and Precautions (5.2)].
Increased Blood Pressure and Heart Rate
Advise patients that QuilliChew ER can elevate blood pressure and heart rate [see Warnings and Precautions (5.3)].
Psychiatric Adverse Reactions
Advise patients that QuilliChew ER, at recommended doses, can cause psychotic or manic symptoms, even in patients without a prior history of psychotic symptoms or mania [see Warnings and Precautions (5.4)].
Priapism
Advise patients, caregivers, and family members of the possibility of painful or prolonged penile erections (priapism). Instruct the patient to seek immediate medical attention in the event of priapism [see Warnings and Precautions (5.5)].
Circulation Problems in Fingers and Toes [Peripheral Vasculopathy, including Raynaud’s Phenomenon]
- Instruct patients beginning treatment with QuilliChew ER about the risk of peripheral vasculopathy, including Raynaud’s phenomenon, and associated signs and symptoms: fingers or toes may feel numb, cool, painful, and/or may change color from pale, to blue, to red.
- Instruct patients to report to their physician any new numbness, pain, skin color change, or sensitivity to temperature in fingers or toes.
- Instruct patients to call their physician immediately with any signs of unexplained wounds appearing on fingers or toes while taking QuilliChew ER.
- Further clinical evaluation (e.g., rheumatology referral) may be appropriate for certain patients [see Warnings and Precautions (5.6)].
Long-Term Suppression of Growth in Pediatric Patients
Advise patients, families, and caregivers that QuilliChew ER can cause slowing of growth and weight loss [see Warnings and Precautions (5.7)].
Alcohol Effect
Advise patients to avoid alcohol while taking QuilliChew ER extended-release chewable tablets. Consumption of alcohol while taking QuilliChew ER may result in a more rapid release of the dose of methylphenidate [see Clinical Pharmacology (12.3)].
Risks in Patients with Phenylketonuria (PKU)
Advise patients with phenylketonuria that QuilliChew ER extended-release chewable tablets contain phenylalanine, a component of aspartame [see Warnings and Precautions (5.8)].
Increased Intraocular Pressure (IOP) and Glaucoma
Advise patients that IOP and glaucoma may occur during treatment with QuilliChew ER [see Warnings and Precautions (5.9)].
Motor and Verbal Tics, and Worsening of Tourette’s Syndrome
Advise patients that motor and verbal tics and worsening of Tourette’s Syndrome may occur during treatment with QuilliChew ER. Instruct patients to notify their healthcare provider if emergence of new tics or worsening of tics or Tourette’s syndrome occurs [see Warnings and Precautions (5.10)].
Pregnancy Registry
Advise patients that there is a pregnancy exposure registry that monitors pregnancy outcomes in females exposed to QuilliChew ER during pregnancy [see Use in Specific Populations (8.1)].
Lactation
Advise nursing mother to monitor infants exposed to QuilliChew ER through breastmilk for agitation, poor feeding, and reduced weight gain [see Use in Specific Populations (8.2)].
This product’s label may have been updated. For current full prescribing information, please visit www.trispharma.com.
Distributed by:

Manufactured by:
Tris Pharma, Inc.
Monmouth Junction, NJ 08852
LB8533
Rev. 02
Medication Guide
|
MEDICATION GUIDE QuilliChew ER®(quil-ih' CHOO' ee-ahr) (methylphenidate hydrochloride) extended-release chewable tablets CII |
|
What is the most important information I should know about QuilliChew ER? QuilliChew ER may cause serious side effects, including:
Your healthcare provider should check you or your child carefully for heart problems before starting QuilliChew ER. Tell your healthcare provider if you or your child have any heart disease, or heart defects. Call your healthcare provider or go to the nearest hospital emergency room right away if you or your child has any signs of heart problems such as chest pain, shortness of breath, or fainting while taking QuilliChew ER.
Your health care provider should check your or your child’s blood pressure and heart rate regularly during treatment with QuilliChew ER. Mental (Psychiatric) problems:
Tell your healthcare provider about any mental problems you or your child have, or about a family history of suicide, bipolar illness, or depression. Call your healthcare provider right away if you or your child have any new or worsening mental symptoms or problems while taking QuilliChew ER, especially seeing or hearing things that are not real, believing things that are not real, or are suspicious. |
|
What is QuilliChew ER? QuilliChew ER is a central nervous system stimulant prescription medicine. QuilliChew ER is an extended-release chewable tablet. It is used for the treatment of Attention Deficit Hyperactivity Disorder (ADHD). QuilliChew ER may help increase attention and decrease impulsiveness and hyperactivity in people with ADHD. It is not known if QuilliChew ER is safe and effective in children under 6 years of age. QuilliChew ER is a federally controlled substance (CII) because it contains methylphenidate that can be a target for people who abuse prescription medicines or street drugs. Keep QuilliChew ER in a safe place to protect it from theft. Never give your QuilliChew ER to anyone else, because it may cause death or harm them. Selling or giving away QuilliChew ER may harm others and is against the law. |
|
Do not take QuilliChew ER if you or your child:
QuilliChew ER may not be right for you or your child. Before starting QuilliChew ER tell your or your child’s health care provider about all health conditions (or a family history of) including:
Tell your health- care provider about all of the medicines that you or your child take including prescription and nonprescription medicines, vitamins, and herbal supplements. QuilliChew ER and some medicines may interact with each other and cause serious side effects. Sometimes the doses of other medicines will need to be adjusted while taking QuilliChew ER. Your health care provider will decide whether QuilliChew ER can be taken with other medicines. Especially tell your healthcare provider if you or your child takes:
Know the medicines that you or your child takes. Keep a list of your medicines with you to show your health care provider and pharmacist. Do not start any new medicine while taking QuilliChew ER without talking to your healthcare provider first. |
|
How should QuilliChew ER be taken?
If you or your child take too much QuilliChew ER, call your healthcare provider or Poison Help line at 1-800-222-1222 or go to the nearest hospital emergency room right away. |
|
What should I avoid while taking QuilliChew ER?
|
|
What are the possible side effects of QuilliChew ER? QuilliChew ER may cause serious side effects, including:
Other serious side effects include:
Tell your healthcare provider if you or your child have numbness, pain, skin color change, or sensitivity to temperature in your fingers or toes, or if you or your child have any signs of unexplained wounds appearing on fingers or toes during treatment with QuilliChew ER.
The most common side effects of QuilliChew ER include:
These are not all the possible side effects of QuilliChew ER. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|
How should I store QUILLICHEW ER?
|
|
General information about the safe and effective use of QuilliChew ER Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use QuilliChew ER for a condition for which it was not prescribed. Do not give QuilliChew ER to other people, even if they have the same condition. It may harm them. You can ask your pharmacist or healthcare provider for information about QuilliChew ER that is written for health care professionals. |
|
What are the ingredients in QuilliChew ER? Active Ingredient: methylphenidate Inactive Ingredients: aspartame, cherry flavor, citric acid, crospovidone, D&C red #30 (for 30 mg strength), D&C red #7 (for 40 mg strength), guar gum, magnesium stearate, mannitol, microcrystalline cellulose, polyvinyl acetate, polyvinyl alcohol, povidone, silicon dioxide, sodium polystyrene sulfonate, talc, triacetin, xanthan gum. |
|
Distributed by:  Manufactured by: Tris Pharma, Inc., Monmouth Junction, NJ 08852 For more information, please contact Tris Pharma, Inc. at (732) 940-0358 or visit the website at www.QuilliChewER.com. This product’s label may have been updated. For current full prescribing information, please visit www.trispharma.com. |
|
This Medication Guide has been approved by the U.S. Food and Drug Administration Revised: 10/2023 |
PRINCIPAL DISPLAY PANEL
NDC 24478-074-01
QUILLICHEW ER
(methylphenidate HCI)
extended-release chewable tablets CII
20 mg
100 Tablets
Rx Only

20mglabel
| QUILLICHEW ER
methylphenidate hydrochloride tablet, chewable, extended release |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| QUILLICHEW ER
methylphenidate hydrochloride tablet, chewable, extended release |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| QUILLICHEW ER
methylphenidate hydrochloride tablet, chewable, extended release |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Labeler - NextWave Pharmaceuticals, Inc (008816703) |
Frequently asked questions
- What are the brands of methylphenidate?
- Jornay PM vs other methylphenidate formulations - how do they compare?
- Is QuilliChew ER a controlled substance?
- What is metilfenidato used for?
- Ritalin vs Vyvanse - What's the difference between them?
- Concerta vs Adderall - What's the difference between them?
- How is Cotempla XR-ODT different to other brands of methylphenidate?
More about QuilliChew ER (methylphenidate)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (2)
- Drug images
- Latest FDA alerts (5)
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- Drug class: CNS stimulants
- Breastfeeding
- En español
Patient resources
Professional resources
Other brands
Concerta, Ritalin, Jornay PM, Ritalin LA, ... +9 more



