WATSON 0140 Pill: white, six-sided, 6mm
Generic Name: ethinyl estradiol/norethindrone
The pill with imprint WATSON 0140 (White, Six-sided, 6mm) has been identified as Tilia Fe ethinyl estradiol 0.02 mg / norethindrone acetate 1 mg and is used for Birth Control, and Acne. It belongs to the drug class contraceptives and is not a controlled substance.
Images for WATSON 0140
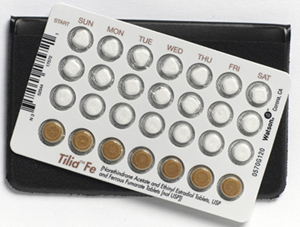

Tilia Fe
- Generic Name
- ethinyl estradiol/norethindrone
- Imprint
- WATSON 0140
- Strength
- ethinyl estradiol 0.02 mg / norethindrone acetate 1 mg
- Color
- White
- Size
- 6.00 mm
- Shape
- Six-sided
- Availability
- Prescription only
- Drug Class
- Contraceptives
- Pregnancy Category
- X - Not for use in pregnancy
- CSA Schedule
- Not a controlled drug
- Labeler / Supplier
- Watson Pharmaceuticals
- National Drug Code (NDC)
- 52544-0143 (Discontinued)
- Inactive Ingredients
-
lactose anhydrous,
magnesium stearate,
microcrystalline cellulose,
sodium starch glycolate type A potato,
polacrilin potassium,
povidone,
D&C Yellow No. 10,
FD&C Blue No. 1,
aluminum oxide
Note: Inactive ingredients may vary.
See also:
More about Tilia Fe (ethinyl estradiol / norethindrone)
- Check interactions
- Compare alternatives
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Drug class: contraceptives
Patient resources
Other brands
Lo Loestrin Fe, Junel Fe 1/20, Aurovela Fe 1/20, Loestrin 21 1/20, ... +46 more
Professional resources
Other brands
Lo Loestrin Fe, Blisovi Fe 1/20, Aurovela Fe 1/20, Loestrin 21 1/20, ... +83 more
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
