Lifestar 807 Pill: black white, capsule/oblong
The pill with imprint Lifestar 807 (Black / White, Capsule/Oblong, 0mm) has been identified as Lansoprazole Delayed-Release 30 mg and is used for Barrett's Esophagus, GERD, Duodenal Ulcer, Duodenal Ulcer Prophylaxis, and Erosive Esophagitis. It belongs to the drug class proton pump inhibitors and is not a controlled substance.
Images for Lifestar 807
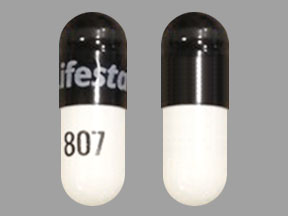
Lansoprazole Delayed-Release
- Imprint
- Lifestar 807
- Strength
- 30 mg
- Color
- Black / White
- Shape
- Capsule/Oblong
- Availability
- Rx and/or OTC
- Drug Class
- Proton pump inhibitors
- Pregnancy Category
- B - No proven risk in humans
- CSA Schedule
- Not a controlled drug
- Labeler / Supplier
- Lifestar Pharma LLC
- Manufacturer
- Inventia Healthcare Private Limited
- National Drug Code (NDC)
See also:
More about lansoprazole
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (144)
- Drug images
- Side effects
- Dosage information
- Patient tips
- During pregnancy
- Support group
- Drug class: proton pump inhibitors
- Breastfeeding
Patient resources
Other brands
Prevacid, Prevacid OTC, Prevacid SoluTab
Professional resources
Other brands
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
