Uzedy Dosage
Generic name: RISPERIDONE 50mg in 0.14mL
Dosage form: injection, suspension, extended release
Drug class: Atypical antipsychotics
Medically reviewed by Drugs.com. Last updated on Jan 24, 2025.
2.1 Recommended Dosage
For patients who have never taken risperidone, establish tolerability with oral risperidone prior to initiating UZEDY.
UZEDY must be administered by a healthcare professional as an abdominal or upper arm subcutaneous injection. Do not administer UZEDY by any other route.
For detailed preparation and administration instructions, see Dosage and Administration (2.4).
To start UZEDY, switch from oral daily risperidone. Initiate UZEDY, as either a once monthly injection or a once every 2 month injection, the day after the last dose of oral therapy. See Table 1 to determine how to switch from oral risperidone to UZEDY once monthly (50 mg, 75 mg, 100 mg, or 125 mg) or once every 2 months (100 mg, 150 mg, 200 mg, or 250 mg) given via abdominal or upper arm subcutaneous injection. Neither a loading dose nor supplemental oral risperidone doses are recommended when switching.
|
Prior Therapy |
UZEDY Dosage Once Monthly |
UZEDY Dosage Once Every 2 Months |
| 2 mg of oral risperidone per day | 50 mg | 100 mg |
| 3 mg of oral risperidone per day | 75 mg | 150 mg |
| 4 mg of oral risperidone per day | 100 mg | 200 mg |
| 5 mg of oral risperidone per day | 125 mg | 250 mg |
Patients can switch between doses of UZEDY once monthly and once every 2 months by administering the first dose of the new dosing regimen on the next scheduled date of administration in the original dosing regimen. Revise the dose administration schedule to reflect the change.
When a dose of UZEDY is missed, administer the next UZEDY injection as soon as possible. Do not administer more frequently than recommended.
2.2 Dosage Modifications in Patients with Renal Impairment or Hepatic Impairment
Prior to initiating UZEDY in patients with renal or hepatic impairment, titrate with oral risperidone to at least 2 mg once daily. Following oral titration, and based on clinical response and tolerability, the recommended dosage of UZEDY is 50 mg once monthly.
Dosage Modifications for Concomitant Use with Strong CYP2D6 Inhibitors and Strong CYP3A4 Inducers
Concomitant Use with Strong CYP2D6 Inhibitors
When initiation of fluoxetine or paroxetine is considered, place patients on a lower dose of UZEDY prior to the planned start of fluoxetine or paroxetine therapy to adjust for the expected increase in plasma concentrations of risperidone.
When fluoxetine or paroxetine is initiated in patients receiving the recommended dose of 50 mg once monthly or 100 mg once every 2 months of UZEDY, continue treatment with these doses unless clinical judgment necessitates interruption of UZEDY.
Concomitant Use with Strong CYP3A4 Inducers
At the initiation of therapy with strong CYP3A4 inducers (such as carbamazepine), patients should be closely monitored during the first 4 to 8 weeks since the dose of UZEDY may need to be adjusted. A dose increase, or additional oral risperidone, may be considered.
On discontinuation of a strong CYP3A4 inducer, re-evaluated the dosage of UZEDY or any additional oral risperidone therapy and, if necessary, decrease to adjust for the expected increase in plasma concentration of risperidone.
On discontinuation of a strong CYP3A4 inducer in a patient treated with UZEDY 50 mg once monthly or 100 mg once every 2 months, continue treatment with these doses unless clinical judgment necessitates interruption of UZEDY.
Preparation and Administration Instructions
- Read the instructions for preparation and administration below before administering UZEDY.
- For subcutaneous injection only. Do not inject by any other route.
- To be administered by a healthcare professional only.
- Allow UZEDY to come to room temperature for at least 30 minutes prior to administration.
- As a universal precaution, always wear gloves.
STEP 1
Check to make sure UZEDY kit contains:
- One sterile single-dose, prefilled glass syringe
- One sterile 21G x 5/8” needle
Do not substitute any components of the kit for administration.


STEP 2
Remove the kit from refrigerated storage and allow the package to sit at room temperature (20°C to 25°C [68°F to 77°F]) for at least 30 minutes.
Note: UZEDY is a solid at refrigerated temperatures and must reach room temperature prior to administration. Do not warm any other way and keep protected from light.

STEP 3
Check that the drug in the syringe is white to off-white, opaque in color, and free from non-white particulate matter. Check that the pouch label states the needle size is 21G x 5/8”.
Do not use if any component of the kit is damaged or if the expiration date has passed.

STEP 4
Expose the safety needle hub by peeling back the paper tab of the needle pouch. Set aside for use in Step 7.
STEP 5
|
IMPORTANT: This step must be performed to ensure complete dosing. UZEDY is viscous and forceful downward flicks are required to move the bubble to the cap of the syringe. Failure to move the bubble to the cap of the syringe could result in incomplete dosage. |
Firmly hold the syringe by the white collar.

Flick Syringe Forcefully Three Times to Move the Bubble to the Cap
- Flick with a forceful downward whipping motion of your full arm to move the bubble to the cap of the syringe.
- Repeat this action 3 times to ensure that the bubble is at the cap of the syringe.
Note: Standing while you do this may help achieve required force.
Check that the Bubble is at the Cap of the Syringe
- The bubble will appear partially opaque.
- Holding the syringe up to light or against a dark backdrop may improve visibility.
- If the bubble is not at the cap, repeat Step 5 until it is.


STEP 6
Hold the syringe vertically by the white collar. Bend and snap off the cap.
Do not touch the syringe tip to avoid contamination.

STEP 7
Attach the Needle to the Syringe
- Hold the syringe vertically with the white collar at the top.
- Push the green hub of safety needle inside the white collar of syringe and rotate the safety needle while holding the white collar until secure and tight.
Inspect the needle connection to check that the hub is not damaged.


STEP 8
Select Injection Site from the Following Areas:
- Stomach area (abdomen) around the belly button
- Back and outer area of the upper arms
Do not inject UZEDY anywhere except in the areas specified above.
Do not inject UZEDY into an area that is tender, red, bruised, callused, tattooed, hard, or has scars or stretch marks.

STEP 9
Clean the Injection Site with an alcohol wipe.
STEP 10
Remove the needle sheath by pulling the needle sheath away from the green hub to expose the needle.
Do not expel any visible air bubble.

STEP 11
Pinch at least 1 inch of the area of cleaned skin with your free hand.

STEP 12
Insert the needle into subcutaneous tissue (actual angle of injection will depend on the amount of subcutaneous tissue). Do not apply pressure to the plunger.

STEP 13
Release the pinched skin once the needle is in the subcutaneous tissue.

STEP 14
Inject the Medication
- Push on the plunger using a slow, firm, and steady push until the entire dose is delivered.
- Inject the entire dose at one time, without interruption.
- Check that the plunger stopper is at the White Collar.
IMPORTANT: UZEDY is viscous. Resistance will be experienced during dose delivery. Do not use excessive force in an attempt to deliver UZEDY faster.


STEP 15
Wait 2-3 seconds after the entire dose is delivered before removing the needle. Slowly pull the needle out from the injection site at the same angle as insertion.

STEP 16
Activate (lock) the safety needle shield using one of the following methods:
- Surface Activation: Place the needle shield on a flat surface and pull the syringe backward until the needle shield covers the needle tip.
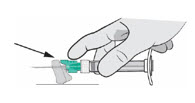
- Finger/Thumb Activation: Press either your thumb or finger on the needle shield and push it forward until the needle shield covers the needle tip.
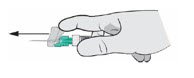
There will be an audible click when the needle safety shield is locked.
Dispose of all syringe components in a suitable sharps container.
Frequently asked questions
- What are the strongest sleeping pills?
- What drugs cause tardive dyskinesia?
- What is the difference between Perseris and Risperdal Consta?
More about Uzedy (risperidone)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Drug images
- Side effects
- During pregnancy
- FDA approval history
- Drug class: atypical antipsychotics
- Breastfeeding
- En español
Patient resources
Other brands
Risperdal, Perseris, Risperdal Consta, Risvan, Rykindo
Professional resources
Other brands
Risperdal, Perseris, Risperdal Consta, Risvan, Rykindo
Related treatment guides
See also:
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.




