Prolia Dosage
Generic name: DENOSUMAB 60mg in 1mL
Dosage form: injection
Drug class: Miscellaneous bone resorption inhibitors
Medically reviewed by Drugs.com. Last updated on May 22, 2025.
Pregnancy Testing Prior to Initiation of Prolia
Pregnancy must be ruled out prior to administration of Prolia. Perform pregnancy testing in all females of reproductive potential prior to administration of Prolia. Based on findings in animals, Prolia can cause fetal harm when administered to pregnant women.
Laboratory Testing in Patients with Advanced Chronic Kidney Disease Prior to Initiation of Prolia
In patients with advanced chronic kidney disease [i.e., estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73 m2], including dialysis-dependent patients, evaluate for the presence of chronic kidney disease mineral and bone disorder (CKD-MBD) with intact parathyroid hormone (iPTH), serum calcium, 25(OH) vitamin D, and 1,25 (OH)2 vitamin D prior to decisions regarding Prolia treatment. Consider also assessing bone turnover status (serum markers of bone turnover or bone biopsy) to evaluate the underlying bone disease that may be present.
Recommended Dosage
Prolia should be administered by a healthcare provider.
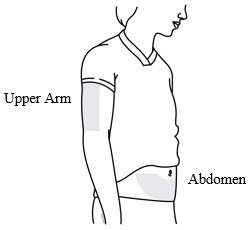
The recommended dose of Prolia is 60 mg administered as a single subcutaneous injection once every 6 months. Administer Prolia via subcutaneous injection in the upper arm, the upper thigh, or the abdomen. All patients should receive calcium 1000 mg daily and at least 400 IU vitamin D daily.
If a dose of Prolia is missed, administer the injection as soon as the patient is available. Thereafter, schedule injections every 6 months from the date of the last injection.
Preparation and Administration
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit. Prolia is a clear, colorless to pale yellow solution that may contain trace amounts of translucent to white proteinaceous particles. Do not use if the solution is discolored or cloudy or if the solution contains many particles or foreign particulate matter.
Prior to administration, Prolia may be removed from the refrigerator and brought to room temperature up to 25°C (77°F) by standing in the original container. This generally takes 15 to 30 minutes. Do not warm Prolia in any other way.
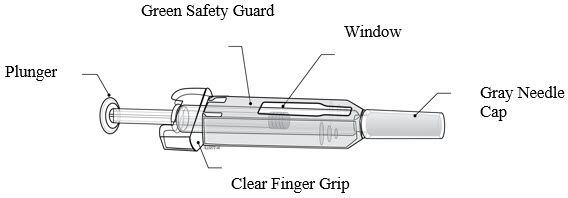
Instructions for Administration of Prolia Prefilled Syringe with Needle Safety Guard
IMPORTANT: In order to minimize accidental needlesticks, the Prolia single-dose prefilled syringe will have a green safety guard; manually activate the safety guard after the injection is given.
DO NOT slide the green safety guard forward over the needle before administering the injection; it will lock in place and prevent injection.
Activate the green safety guard (slide over the needle) after the injection.
Step 1: Remove Gray Needle Cap
| Remove needle cap. | 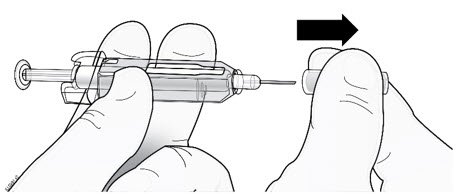 |
Step 2: Administer Subcutaneous Injection
| Choose an injection site. The recommended injection sites for Prolia include: the upper arm OR the upper thigh OR the abdomen. | 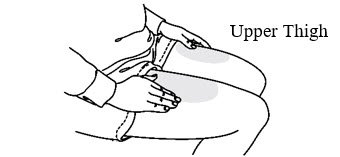 |
|
 |
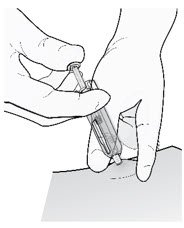
| Insert needle and inject all the liquid subcutaneously. Do not administer into muscle or blood vessel. |
 |
DO NOT put gray needle cap back on needle.
Step 3: Immediately Slide Green Safety Guard Over Needle
With the needle pointing away from you.
Hold the prefilled syringe by the clear finger grip with one hand. Then, with the other hand, grasp the green safety guard by its base and gently slide it towards the needle until the green safety guard locks securely in place and/or you hear a "click". DO NOT grip the green safety guard too firmly - it will move easily if you hold and slide it gently.
| Hold clear finger grip. | 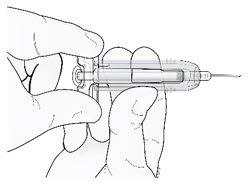 |
|
| Gently slide green safety guard over needle and lock securely in place. Do not grip green safety guard too firmly when sliding over needle. | 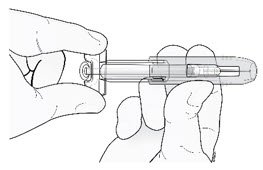 |
Immediately dispose of the syringe and needle cap in the nearest sharps container. DO NOT put the needle cap back on the used syringe.
Frequently asked questions
- What's the difference between Prolia and Reclast?
- Does Prolia weaken your immune system?
- How do you give a Prolia injection?
- Does Prolia cause weight gain?
- Evenity vs Prolia: Which is right for you?
- Does Prolia increase bone density?
- Xgeva vs Prolia. How do they compare?
- Can you drink alcohol while taking Prolia?
- How many years should you take Prolia?
More about Prolia (denosumab)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (377)
- Drug images
- Latest FDA alerts (2)
- Side effects
- Patient tips
- During pregnancy
- Support group
- FDA approval history
- Drug class: miscellaneous bone resorption inhibitors
- Breastfeeding
- En español
Patient resources
Other brands
Professional resources
Other brands
Xgeva, Jubbonti, Wyost, Osenvelt, ... +3 more
Related treatment guides
See also:
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.