Molindone: Package Insert / Prescribing Info
Package insert / product label
Generic name: molindone hydrochloride
Dosage form: tablet
Drug class: Miscellaneous antipsychotic agents
Medically reviewed by Drugs.com. Last updated on Jan 2, 2024.
On This Page
WARNING
Increased Mortality in Elderly Patients with Dementia-Related Psychosis – Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of seventeen placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. Molindone Hydrochloride Tablets, USP are not approved for the treatment of patients with dementia-related psychosis (see WARNINGS).
Molindone Description
Molindone Hydrochloride is a dihydroindolone compound which is not structurally related to the phenothiazines, the butyrophenones or the thioxanthenes.
Molindone Hydrochloride is 3-ethyl-6, 7-dihydro-2-methyl-5-(morpholinomethyl) indol-4(5H)-one hydrochloride. It is a white to off-white or pale-pink crystalline powder, freely soluble in water and alcohol.
Molindone Hydrochloride Tablets, USP contain the following inactive ingredients: alginic acid, calcium sulfate, colloidal silicon dioxide, lactose monohydrate, magnesium stearate, microcrystalline cellulose, povidone and sodium starch glycolate.
Colors: 5 mg contains FD&C Yellow #6 Aluminum Lake
- 10 mg contains FD&C Blue #2 Aluminum Lake
- 25 mg contains FD&C Blue #2 Aluminum Lake, FD&C Yellow #6, Aluminum Lake and D&C Yellow #10 Aluminum Lake
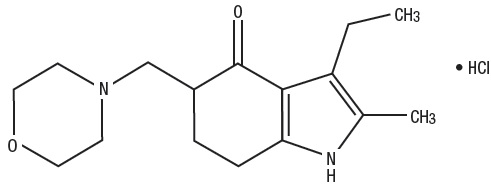
Molindone Hydrochloride is represented by the following structural formula:
The empirical formula is C16H24N2O2•HCl representing a molecular weight of 312.83.
Molindone - Clinical Pharmacology
Molindone Hydrochloride has a pharmacological profile in laboratory animals which predominantly resembles that of other antipsychotic agents causing reduction of spontaneous locomotion and aggressiveness, suppression of a conditioned response and antagonism of the bizarre stereotyped behavior and hyperactivity induced by amphetamines. In addition, Molindone Hydrochloride antagonizes the depression caused by the tranquilizing agent tetrabenazine.
In human clinical studies an antipsychotic effect is achieved in the absence of muscle relaxing or incoordinating effects. Based on EEG studies, Molindone Hydrochloride exerts its effect on the ascending reticular activating system.
Human metabolic studies show Molindone Hydrochloride Tablets to be rapidly absorbed and metabolized when given orally. Unmetabolized drug reached a peak blood level at 1.5 hours. Pharmacological effect from a single oral dose persists for 24 to 36 hours. There are 36 recognized metabolites with less than 2 to 3% unmetabolized Molindone Hydrochloride Tablets being excreted in urine and feces.
Indications and Usage for Molindone
Molindone Hydrochloride Tablets, USP are indicated for the management of schizophrenia. The efficacy of Molindone Hydrochloride Tablets, USP in schizophrenia was established in clinical studies which enrolled newly hospitalized and chronically hospitalized, acutely ill, schizophrenic patients as subjects.
Contraindications
Molindone Hydrochloride Tablets are contraindicated in severe central nervous system depression (alcohol, barbiturates, narcotics, etc.) or comatose states, and in patients with known hypersensitivity to the drug.
Warnings
Increased Mortality in Elderly Patients with Dementia-Related Psychosis — Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death.
Molindone Hydrochloride Tablets are not approved for the treatment of patients with dementia-related psychosis (see BOXED WARNING).
Tardive Dyskinesia
Tardive dyskinesia, a syndrome consisting of potentially irreversible, involuntary, dyskinetic movements may develop in patients treated with antipsychotic drugs. Although the prevalence of the syndrome appears to be highest among the elderly, especially elderly women, it is impossible to rely upon prevalence estimates to predict, at the inception of antipsychotic treatment, which patients are likely to develop the syndrome. Whether antipsychotic drug products differ in their potential to cause tardive dyskinesia is unknown.
Both the risk of developing the syndrome and the likelihood that it will become irreversible are believed to increase as the duration of treatment and the total cumulative dose of antipsychotic drugs administered to the patient increase. However, the syndrome can develop, although much less commonly, after relatively brief treatment periods at low doses.
There is no known treatment for established cases of tardive dyskinesia, although the syndrome may remit, partially or completely, if antipsychotic treatment is withdrawn. Antipsychotic treatment, itself, however, may suppress (or partially suppress) the signs and symptoms of the syndrome and thereby may possibly mask the underlying disease process. The effect that symptomatic suppression has upon the long-term course of the syndrome is unknown.
Given these considerations, antipsychotics should be prescribed in a manner that is most likely to minimize the occurrence of tardive dyskinesia. Chronic antipsychotic treatment should generally be reserved for patients who suffer from a chronic illness that, 1) is known to respond to antipsychotic drugs, and 2) for whom alternative, equally effective, but potentially less harmful treatments are not available or appropriate. In patients who do require chronic treatment, the smallest dose and the shortest duration of treatment producing a satisfactory clinical response should be sought. The need for continued treatment should be reassessed periodically.
If signs and symptoms of tardive dyskinesia appear in a patient on antipsychotics, drug discontinuation should be considered. However, some patients may require treatment despite the presence of the syndrome.
(For further information about the description of tardive dyskinesia and its clinical detection, please refer to the section on ADVERSE REACTIONS.)
Falls
Molindone Hydrochloride may cause somnolence, postural hypotension, motor and sensory instability, which may lead to falls and, consequently, fractures or other injuries. For patients with diseases, conditions, or medications that could exacerbate these effects, complete fall risk assessments when initiating antipsychotic treatment and recurrently for patients on long-term antipsychotic therapy.
Neuroleptic Malignant Syndrome (NMS)
A potentially fatal symptom complex sometimes referred to as Neuroleptic Malignant Syndrome (NMS) has been reported in association with antipsychotic drugs. Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, altered mental status and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac dysrhythmias).
The diagnostic evaluation of patients with this syndrome is complicated. In arriving at a diagnosis, it is important to identify cases where the clinical presentation includes both serious medical illness (e.g., pneumonia, systemic infection, etc.) and untreated or inadequately treated extrapyramidal signs and symptoms (EPS). Other important considerations in the differential diagnosis include central anticholinergic toxicity, heat stroke, drug fever and primary central nervous system (CNS) pathology.
The management of NMS should include, 1) immediate discontinuation of antipsychotic drugs and other drugs not essential to concurrent therapy, 2) intensive symptomatic treatment and medical monitoring, and 3) treatment of any concomitant serious medical problems for which specific treatments are available. There is no general agreement about specific pharmacological treatment regimens for uncomplicated NMS.
If a patient requires antipsychotic drug treatment after recovery from NMS, the potential reintroduction of drug therapy should be carefully considered. The patient should be carefully monitored, since recurrences of NMS have been reported.
Precautions
General
Some patients receiving Molindone Hydrochloride may note drowsiness initially and they should be advised against activities requiring mental alertness until their response to the drug has been established.
Increased activity has been noted in patients receiving Molindone Hydrochloride. Caution should be exercised where increased activity may be harmful.
Molindone Hydrochloride does not lower the seizure threshold in experimental animals to the degree noted with more sedating antipsychotic drugs. However, in humans convulsive seizures have been reported in a few instances.
The physician should be aware that this tablet preparation contains calcium sulfate as an excipient and that calcium ions may interfere with the absorption of preparations containing phenytoin sodium and tetracyclines.
Molindone Hydrochloride has an antiemetic effect in animals. A similar effect may occur in humans and may obscure signs of intestinal obstruction or brain tumor.
Antipsychotic drugs elevate prolactin levels; the elevation persists during chronic administration. Tissue culture experiments indicate that approximately one-third of human breast cancers are prolactin dependent in vitro, a factor of potential importance if the prescription of these drugs is contemplated in a patient with a previously detected breast cancer. Although disturbances such as galactorrhea, amenorrhea, gynecomastia, and impotence have been reported, the clinical significance of elevated serum prolactin levels is unknown for most patients. An increase in mammary neoplasms has been found in rodents after chronic administration of antipsychotic drugs. Neither clinical studies nor epidemiologic studies conducted to date, however, have shown an association between chronic administration of these drugs and mammary tumorigenesis; the available evidence is considered too limited to be conclusive at this time.
Molindone Hydrochloride has not been shown effective in the management of behavioral complications in patients with mental retardation.
Leukopenia, Neutropenia and Agranulocytosis
Some patients receiving Molindone Hydrochloride may note drowsiness initially and they should be advised against activities requiring mental alertness until their response to the drug has been established.
Increased activity has been noted in patients receiving Molindone Hydrochloride. Caution should be exercised where increased activity may be harmful.
Molindone Hydrochloride does not lower the seizure threshold in experimental animals to the degree noted with more sedating antipsychotic drugs. However, in humans convulsive seizures have been reported in a few instances.
The physician should be aware that this tablet preparation contains calcium sulfate as an excipient and that calcium ions may interfere with the absorption of preparations containing phenytoin sodium and tetracyclines.
Molindone Hydrochloride has an antiemetic effect in animals. A similar effect may occur in humans and may obscure signs of intestinal obstruction or brain tumor.
Antipsychotic drugs elevate prolactin levels; the elevation persists during chronic administration. Tissue culture experiments indicate that approximately one-third of human breast cancers are prolactin dependent in vitro, a factor of potential importance if the prescription of these drugs is contemplated in a patient with a previously detected breast cancer. Although disturbances such as galactorrhea, amenorrhea, gynecomastia, and impotence have been reported, the clinical significance of elevated serum prolactin levels is unknown for most patients. An increase in mammary neoplasms has been found in rodents after chronic administration of antipsychotic drugs. Neither clinical studies nor epidemiologic studies conducted to date, however, have shown an association between chronic administration of these drugs and mammary tumorigenesis; the available evidence is considered too limited to be conclusive at this time.
Molindone Hydrochloride has not been shown effective in the management of behavioral complications in patients with mental retardation.
Leukopenia, Neutropenia and Agranulocytosis
Class Effect: In clinical trial and/or postmarketing experience, events of leukopenia/neutropenia and agranulocytosis have been reported temporally related to antipsychotic agents.
Possible risk factors for leukopenia/neutropenia include preexisting low white blood cell count (WBC) and history of drug induced leukopenia/neutropenia. Patients with a history of a clinically significant low WBC or drug induced leukopenia/neutropenia should have their complete blood count (CBC) monitored frequently during the first few months of therapy and discontinuation of Molindone Hydrochloride should be considered at the first sign of a clinically significant decline in WBC in the absence of other causative factors.
Patients with clinically significant neutropenia should be carefully monitored for fever or other symptoms or signs of infection and treated promptly if such symptoms or signs occur. Patients with severe neutropenia (absolute neutrophil count <1000/mm3) should discontinue Molindone Hydrochloride and have their WBC followed until recovery.
Drug Interactions
Potentiation of drugs administered concurrently with Molindone Hydrochloride has not been reported. Additionally, animal studies have not shown increased toxicity when Molindone Hydrochloride is given concurrently with representative members of three classes of drugs (i.e., barbiturates, chloral hydrate and antiparkinson drugs).
Pregnancy
Studies in pregnant patients have not been carried out. Reproduction studies have been performed in the following animals:
Pregnant Rats oral dose—
no adverse effect 20 mg/kg/day - 10 days
no adverse effect 40 mg/kg/day - 10 days
Pregnant Mice oral dose—
slight increase resorptions 20 mg/kg/day - 10 days
slight increase resorptions 40 mg/kg/day - 10 days
Pregnant Rabbits oral dose—
no adverse effect 5 mg/kg/day - 12 days
no adverse effect 10 mg/kg/day - 12 days
no adverse effect 20 mg/kg/day - 12 days
Animal reproductive studies have not demonstrated a teratogenic potential. The anticipated benefits must be weighed against the unknown risks to the fetus if used in pregnant patients.
Non-teratogenic Effects
Neonates exposed to antipsychotic drugs, during the third trimester of pregnancy are at risk for extrapyramidal and/or withdrawal symptoms following delivery. There have been reports of agitation, hypertonia, hypotonia, tremor, somnolence, respiratory distress and feeding disorder in these neonates. These complications have varied in severity; while in some cases symptoms have been self-limited, in other cases neonates have required intensive care unit support and prolonged hospitalization.
Molindone Hydrochloride should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Adverse Reactions/Side Effects
CNS Effects
The most frequently occurring effect is initial drowsiness that generally subsides with continued usage of the drug or lowering of the dose.
Noted less frequently were depression, hyperactivity and euphoria.
Extrapyramidal Symptoms
Extrapyramidal symptoms noted below may occur in susceptible individuals and are usually reversible with appropriate management.
Parkinson Syndrome
Akinesia, characterized by rigidity, immobility and reduction of voluntary movements and tremor, have been observed. Occurrence is less frequent than akathisia.
Dystonia
Class effect: Symptoms of dystonia, prolonged abnormal contractions of muscle groups, may occur in susceptible individuals during the first few days of treatment. Dystonic symptoms include: spasm of the neck muscles, sometimes progressing to tightness of the throat, swallowing difficulty, difficulty breathing, and/or protrusion of the tongue. While these symptoms can occur at low doses, they occur more frequently and with greater severity with high potency and at higher doses of first generation antipsychotic drugs. An elevated risk of acute dystonia is observed in males and younger age groups.
Tardive Dyskinesia
Antipsychotic drugs are known to cause a syndrome of dyskinetic movements commonly referred to as tardive dyskinesia. The movements may appear during treatment or upon withdrawal of treatment and may be either reversible or irreversible (i.e., persistent) upon cessation of further antipsychotic administration.
The syndrome is known to have a variable latency for development and the duration of the latency cannot be determined reliably. It is thus wise to assume that any antipsychotic agent has the capacity to induce the syndrome and act accordingly until sufficient data has been collected to settle the issue definitively for a specific drug product. In the case of antipsychotics known to produce the irreversible syndrome, the following has been observed.
Tardive dyskinesia has appeared in some patients on long-term therapy and has also appeared after drug therapy has been discontinued. The risk appears to be greater in elderly patients on high-dose therapy, especially females. The symptoms are persistent and in some patients appear to be irreversible. The syndrome is characterized by rhythmical involuntary movements of the tongue, face, mouth or jaw (e.g., protrusion of tongue, puffing of cheeks, puckering of mouth, chewing movements). There may be involuntary movements of extremities.
There is no known effective treatment of tardive dyskinesia; antiparkinsonism agents usually do not alleviate the symptoms of this syndrome. It is suggested that all antipsychotic agents be discontinued if these symptoms appear. Should it be necessary to reinstitute treatment, or increase the dosage of the agent, or switch to a different antipsychotic agent, the syndrome may be masked. It has been reported that fine vermicular movements of the tongue may be an early sign of the syndrome and if the medication is stopped at that time the syndrome may not develop (See WARNINGS).
Autonomic Nervous System
Occasionally blurring of vision, tachycardia, nausea, dry mouth and salivation have been reported. Urinary retention and constipation may occur particularly if anticholinergic drugs are used to treat extrapyramidal symptoms. One patient being treated with Molindone Hydrochloride experienced priapism which required surgical intervention, apparently resulting in residual impairment of erectile function.
Laboratory Tests
There have been rare reports of leucopenia and leucocytosis. If such reactions occur, treatment with Molindone Hydrochloride may continue if clinical symptoms are absent. Alterations of blood glucose, B.U.N., and red blood cells have not been considered clinically significant.
Metabolic and Endocrine Effects
Alteration of thyroid function has not been significant. Amenorrhea has been reported infrequently. Resumption of menses in previously amenorrheic women has been reported. Initially heavy menses may occur. Galactorrhea and gynecomastia have been reported infrequently. Increase in libido has been noted in some patients. Impotence has not been reported. Although both weight gain and weight loss have been in the direction of normal or ideal weight, excessive weight gain has not occurred with Molindone Hydrochloride.
Hepatic Effects
There have been rare reports of clinically significant alterations in liver function in association with Molindone Hydrochloride use.
Cardiovascular
Rare, transient, non-specific T wave changes have been reported on E.K.G. Association with a clinical syndrome has not been established. Rarely has significant hypotension been reported.
Ophthalmological
Lens opacities and pigmentary retinopathy have not been reported where patients have received Molindone Hydrochloride. In some patients, phenothiazine induced lenticular opacities have resolved following discontinuation of the phenothiazine while continuing therapy with Molindone Hydrochloride.
Skin
Early, non-specific skin rash, probably of allergic origin, has occasionally been reported. Skin pigmentation has not been seen with Molindone Hydrochloride usage alone.
Molindone Hydrochloride has certain pharmacological similarities to other antipsychotic agents. Because adverse reactions are often extensions of the pharmacological activity of a drug, all of the known pharmacological effects associated with other antipsychotic drugs should be kept in mind when Molindone Hydrochloride is used. Upon abrupt withdrawal after prolonged high dosage an abstinence syndrome has not been noted.
Related/similar drugs
Overdosage
Symptomatic, supportive therapy should be the rule.
Gastric lavage is indicated for the reduction of absorption of Molindone Hydrochloride which is freely soluble in water.
Since the adsorption of Molindone Hydrochloride by activated charcoal has not been determined, the use of this antidote must be considered of theoretical value.
Emesis in a comatose patient is contraindicated. Additionally, while the emetic effect of apomorphine is blocked by Molindone Hydrochloride in animals, this blocking effect has not been determined in humans.
A significant increase in the rate of removal of unmetabolized Molindone Hydrochloride from the body by forced diuresis, peritoneal or renal dialysis would not be expected. (Only 2% of a single ingested dose of Molindone Hydrochloride is excreted unmetabolized in the urine). However, poor response of the patient may justify use of these procedures.
While the use of laxatives or enemas might be based on general principles, the amount of unmetabolized Molindone Hydrochloride in feces is less than 1%. Extrapyramidal symptoms have responded to the use of Diphenhydramine (Benadryl®)*, Amantadine HCl (Symmetrel®)† and the synthetic anticholinergic antiparkinson agents, (i.e., Artane®‡, Cogentin®§, Akineton®¶).
Molindone Dosage and Administration
Initial and maintenance doses of Molindone Hydrochloride Tablets should be individualized.
Initial Dosage Schedule
The usual starting dosage is 50 to 75 mg/day.
—Increase to 100 mg/day in 3 or 4 days.
- —Based on severity of symptomatology, dosage may be titrated up or down depending on individual patient response.
—An increase to 225 mg/day may be required in patients with severe symptomatology.
- Elderly and debilitated patients should be started on lower dosage.
How is Molindone supplied
Molindone Hydrochloride Tablets USP, 5 mg are light orange to orange, oval-shaped tablets, debossed "Є336" on one side and plain on the other side. They are supplied as follows:
Bottles of 100 NDC 42806-336-01
Molindone Hydrochloride Tablets USP, 10 mg are light blue to blue, oval-shaped tablets, debossed "Є337" on one side and plain on the other side. They are supplied as follows:
Bottles of 100 NDC 42806-337-01
Molindone Hydrochloride Tablets USP, 25 mg are light green to green, oval-shaped tablet, debossed "Є338" on one side and bisect on the other side. They are supplied as follows:
Bottles of 100 NDC 42806-338-01
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
Dispense in a tight, light-resistant container as defined in the USP, with a child-resistant closure (as required).
KEEP TIGHTLY CLOSED
*Benadryl is a registered trademark of Warner-Lambert.
†Symmetrel is a registered trademark of Endo Pharmaceuticals Inc.
‡Artane is a registered trademark of Lederle Laboratories.
§Cogentin is a registered trademark of Merck & Co., Inc.
¶Akineton is a registered trademark of Knoll Laboratories.
Distributed by:
Epic Pharma, LLC
Laurelton NY, 11413
Rev. 07-2023-00
MF336REV07/23
OE2798
| MOLINDONE HYDROCHLORIDE
molindone hydrochloride tablet |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| MOLINDONE HYDROCHLORIDE
molindone hydrochloride tablet |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| MOLINDONE HYDROCHLORIDE
molindone hydrochloride tablet |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - EPIC PHARMA, LLC (827915443) |
| Registrant - EPIC PHARMA, LLC (827915443) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| EPIC PHARMA, LLC | 827915443 | MANUFACTURE(42806-336, 42806-337, 42806-338) | |
More about molindone
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (1)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Drug class: miscellaneous antipsychotic agents
- Breastfeeding
- En español