Anti-Diarrheal Tablet: Package Insert / Prescribing Info
Package insert / product label
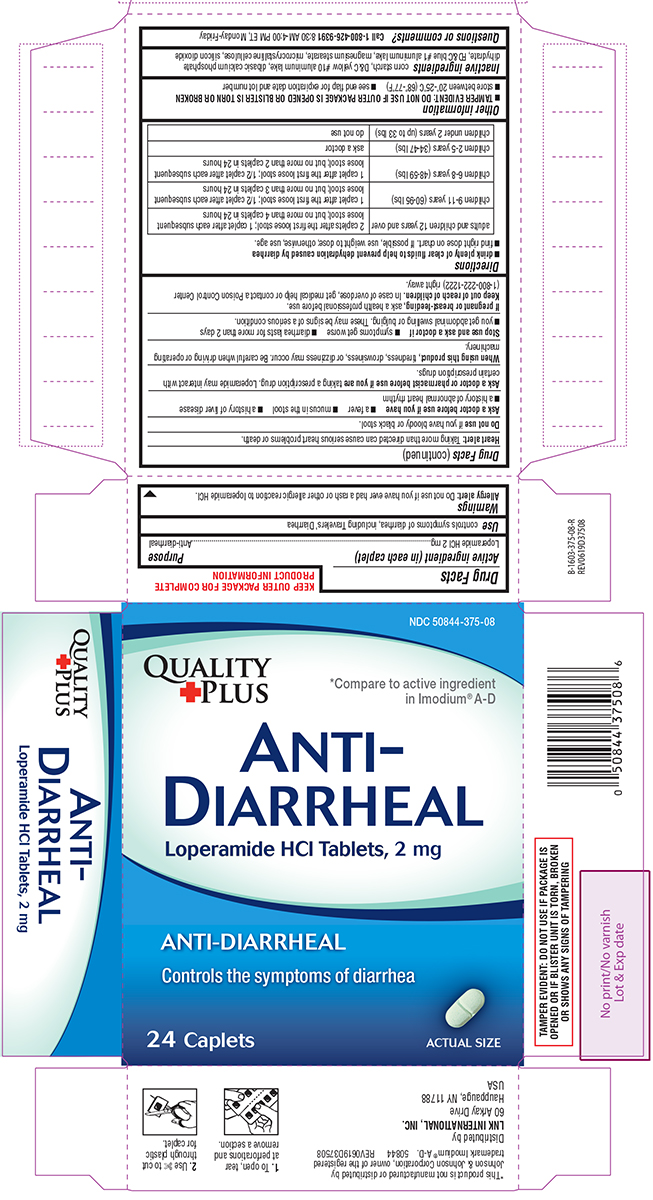
Generic name: loperamide hydrochloride
Dosage form: tablet
Drug class: Antidiarrheals
Medically reviewed by Drugs.com. Last updated on Aug 14, 2025.
Indications and Usage for Anti-Diarrheal Tablet
controls symptoms of diarrhea, including Travelers’ Diarrhea
Warnings
Allergy alert: Do not use if you have ever had a rash or other allergic reaction to loperamide HCl.
Heart alert: Taking more than directed can cause serious heart problems or death.
Ask a doctor before use if you have
- a fever
- mucus in the stool
- a history of liver disease
- a history of abnormal heart rhythm
Ask a doctor or pharmacist before use if you are
taking a prescription drug. Loperamide may interact with certain prescription drugs.
When using this product,
tiredness, drowsiness, or dizziness may occur. Be careful when driving or operating machinery.
Anti-Diarrheal Tablet Dosage and Administration
- drink plenty of clear fluids to help prevent dehydration caused by diarrhea
- find right dose on chart. If possible, use weight to dose; otherwise, use age.
| adults and children 12 years and over | 2 caplets after the first loose stool; 1 caplet after each subsequent loose stool; but no more than 4 caplets in 24 hours |
| children 9-11 years (60-95 lbs) | 1 caplet after the first loose stool; 1/2 caplet after each subsequent loose stool; but no more than 3 caplets in 24 hours |
| children 6-8 years (48-59 lbs) | 1 caplet after the first loose stool; 1/2 caplet after each subsequent loose stool; but no more than 2 caplets in 24 hours |
| children 2-5 years (34-47 lbs) | ask a doctor |
| children under 2 years (up to 33 lbs) | do not use |
Related/similar drugs
Storage and Handling
- TAMPER EVIDENT: DO NOT USE IF OUTER PACKAGE IS OPENED OR BLISTER IS TORN OR BROKEN
- store between 20º-25ºC (68º-77ºF)
- see end flap for expiration date and lot number
Inactive ingredients
corn starch, D&C yellow #10 aluminum lake, dibasic calcium phosphate dihydrate, FD&C blue #1 aluminum lake, magnesium stearate, microcrystalline cellulose, silicon dioxide
Principal Display Panel
QUALITY
+ PLUS
NDC 50844-375-08
*Compare to active ingredient
in Imodium® A-D
ANTI-
DIARRHEAL
Loperamide HCl Tablets, 2 mg
ANTI-DIARRHEAL
Controls the symptoms of diarrhea
24 Caplets
ACTUAL SIZE
TAMPER EVIDENT: DO NOT USE IF PACKAGE IS OPENED OR IF BLISTER UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING
*This product is not manufactured or distributed by Johnson & Johnson Corporation, owner of the registered trademark Imodium® A-D.
50844 REV0619D37508
Distributed by
LNK INTERNATIONAL, INC.
60 Arkay Drive
Hauppauge, NY 11788
USA
Quality Plus 44-375
| ANTI-DIARRHEAL
loperamide hcl tablet |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - L.N.K. International, Inc. (038154464) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867837 | manufacture(50844-375) , pack(50844-375) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 117025878 | manufacture(50844-375) | |
Frequently asked questions
More about loperamide
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (94)
- Drug images
- Latest FDA alerts (3)
- Side effects
- Dosage information
- Patient tips
- During pregnancy
- Drug class: antidiarrheals
- Breastfeeding
- En español
Patient resources
Professional resources
Other brands
Imodium, Imodium A-D, Anti-Diarrheal, Up and Up Anti-Diarrheal Solution

