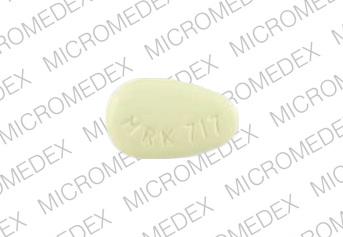
R 717 Pill: yellow, round, 9mm
The pill with imprint R 717 (Yellow, Round, 9mm) has been identified as Diclofenac Sodium Extended Release 100 mg and is used for Back Pain, Ankylosing Spondylitis, Frozen Shoulder, Chronic Pain, and Aseptic Necrosis. It belongs to the drug class Nonsteroidal anti-inflammatory drugs and is not a controlled substance.
Images for R 717

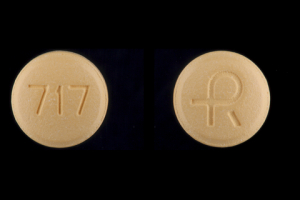
Diclofenac Sodium Extended Release
- Imprint
- R 717
- Strength
- 100 mg
- Color
- Yellow
- Size
- 9.00 mm
- Shape
- Round
- Availability
- Prescription only
- Drug Class
- Nonsteroidal anti-inflammatory drugs
- Pregnancy Category
- D - Positive evidence of risk
- CSA Schedule
- Not a controlled drug
- Labeler / Supplier
- Actavis
Labelers / Repackagers
| NDC Code | Labeler / Repackager |
|---|---|
| 00228-2717 (Discontinued) | Actavis Pharma, Inc. |
| 63874-1055 (Discontinued) | Altura Pharmaceuticals Inc. (repackager) |
Related images for "R 717"
More about diclofenac
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (702)
- Drug images
- Latest FDA alerts (11)
- Side effects
- Dosage information
- Patient tips
- During pregnancy
- Support group
- Drug class: Nonsteroidal anti-inflammatory drugs
- Breastfeeding
- En español
Patient resources
- Diclofenac drug information
- Diclofenac (Intravenous) (Advanced Reading)
- Diclofenac (Oral) (Advanced Reading)
Other brands
Voltaren, Cataflam, Cambia, Voltaren-XR, ... +4 more
Professional resources
- Diclofenac (Systemic, Local) monograph
- Diclofenac (FDA)
- Diclofenac Capsule (FDA)
- Diclofenac Delayed Release Tablets (FDA)
- Diclofenac Extended Release Tablets 100mg (FDA)
Other brands
Voltaren, Cataflam, Cambia, Voltaren-XR, ... +3 more
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.