Dysport Dosage
Generic name: BOTULINUM TOXIN TYPE A 300U
Dosage form: injection, powder, lyophilized, for solution
Drug class: Skeletal muscle relaxants
Medically reviewed by Drugs.com. Last updated on Oct 24, 2024.
2.1 Instructions for Safe Use
The potency units of DYSPORT are not interchangeable with other preparations of botulinum toxin products and, therefore, units of biological activity of DYSPORT cannot be compared to or converted into units of any other botulinum toxin products assessed with any other specific assay method [ see Warnings and Precautions (5.2) and Description (11)]. Reconstituted DYSPORT is intended for intramuscular injection only.
Preparation of Dysport Solution for Administration
DYSPORT is supplied as a dry powder, in single-dose 300 Unit and 500 Unit vials, which must be reconstituted with preservative-free 0.9% Sodium Chloride Injection, USP using aseptic technique prior to intramuscular injection. Table 1 provides dilution instructions for the 300 Unit and 500 Unit vials, depending on the desired final concentration. The desired final concentration after dilution varies depending on the indication (see Table 2for the recommended solution concentration after dilution).
|
|||
| Diluent* per 500 Unit Vial | Resulting Dose Unites per 0.1 mL | DIluent *per 300 Unit Vial | Resulting Dose Units per 0.1 mL |
| 1 mL | 50 Units | 0.6 mL | 50 Units |
| 2 mL | 25 Units | -- | -- |
| 2.5 mL | 20 Units | 1.5 mL | 20 Units |
| -- | -- | 2.5 mL | 12 Units |
| 5 mL ‡ | 10 Units | 3 mL | 10 Units |
Note: These dilutions are calculated for an injection volume of 0.1 mL. A decrease or increase in the DYSPORT dose is also possible by administering a smaller or larger injection volume (i.e., 0.05 mL (50% decrease in dose), 0.08% (20% decrease in dose) or 0.15 mL (50% increase in dose)).
‡ When using 5 mL of diluent for a 500 Unit vial of DYSPORT, no more than 2.5 mL of 0.9% Sodium Chloride Injection, USP should be introduced into the vial. Complete the following steps:
- Reconstitute a 500 Unit vial of DYSPORT with 2.5 mL of Preservative-free 0.9% Sodium Chloride Injection, USP, gently mix, and set the vital aside.
- Withdraw 2.5 mL of Preservative-free 0.9 Sodium Chloride Infection, USP into a 5 mL syringe
- Take the 5 mL syringe with 2.5 ml Preservative-free Sodium Chloride Injection, USP and draw up the DYSPORT solution from the reconstituted vial without inverting and mix gently. The resulting concentration will be 10 units/0.1 mL
- Use immediately after reconstitution in the syringe. Dispose of any unused 0.9% Sodium Chloride Injection, USP.
Using an appropriately sized sterile syringe, needle and aseptic technique, draw up the required amount of sterile, preservative-free 0.9% Sodium Chloride Injection, USP (see Table 1). Insert the needle into the DYSPORT vial. The partial vacuum will begin to pull the saline into the vial. Any remaining required saline should be expressed into the vial manually. Do not use the vial if no partial vacuum is observed. Swirl gently to dissolve. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration.
Reconstituted DYSPORT should be a clear, colorless solution, free of particulate matter, otherwise it should not be injected. Expel any air bubbles in the syringe barrel. Remove the needle used to reconstitute the product and attach an appropriately sized new sterile needle for injection.
After reconstitution, DYSPORT should be used for only one injection session and for only one patient. Discard any unused portion. Once reconstituted, unused DYSPORT may be stored in the original container, in a refrigerator at 2°C to 8°C (36°F to 46°F), protected from light for up to 24 hours until time of use. It must be discarded if not used within 24 hours. Do not freeze reconstituted DYSPORT. Discard the vial and needle in accordance with local regulations.
| Indication | Recommended Concentration | Recommended DYSPORT Dose |
| Cervical Dystonia, Adults | 50 Units/0.1 mL or 25 Units/0.1 mL |
500 Units to 1000 Units |
| Glabellar Lines, Adults | 12 Units/0.1 mL or 20 Units/0.1 mL |
50 Units, divided in five equal aliquots of 10 Units (0.08 mL) each or 50 Units, divided in five equal aliquots of 10 Units (0.05 mL) each |
| Spasticity, Adults* |
10 Units/0.1 mL or 20 Units/0.1 mL |
Upper Limb: 500 Units to 1000 Units Lower Limb: 1000 Units to 1500 Units Maximum total dose per treatment session = 1500 Units |
| Spasticity, Pediatric Patients ‡ |
20 Units/0.1 mL or 50 Units/0.1 mL** |
Upper Limb: 8 Units/kg to 16 Units/kg per limb
|
* No more than 1 mL should generally be administered at any single injections site
‡No more than 0.5 mL of DYSPORT should be administered in any single injection site
** Further dilution with preservative-free 0.9% Sodium Chloride Injection, USP, may be required to achieve the final volume for injection.
Dosing in Cervical Dystonia
The recommended initial dose of DYSPORT for the treatment of cervical dystonia in adults is 500 Units given intramuscularly as a divided dose among affected muscles in patients with or without a history of prior treatment with botulinum toxin. (A description of the average DYSPORT dose and percentage of total dose injected into specific muscles in the pivotal clinical trials can be found in Table 15 of Section 14.1, Clinical Studies – Cervical Dystonia.) Limiting the dose injected into the sternocleidomastoid muscle may reduce the occurrence of dysphagia. Clinical studies with DYSPORT in cervical dystonia suggest that the peak effect occurs between two and four weeks after injection. Simultaneous guided injection of DYSPORT with EMG and/or ultrasound may be helpful in locating active muscles.
Dose Modification
Where dose modification is necessary for the treatment of cervical dystonia, uncontrolled open-label studies suggest that dose adjustment can be made in 250 Unit steps according to the individual patient's response, with re-treatment every 12 weeks or longer, as necessary, based on return of clinical symptoms. Uncontrolled open-label studies also suggest that the total dose administered in a single treatment should be between 250 Units and 1000 Units. Re-treatment, if needed, should not occur in intervals of less than 12 weeks. Doses above 1000 Units have not been systematically evaluated.
Dosing in Glabellar Lines
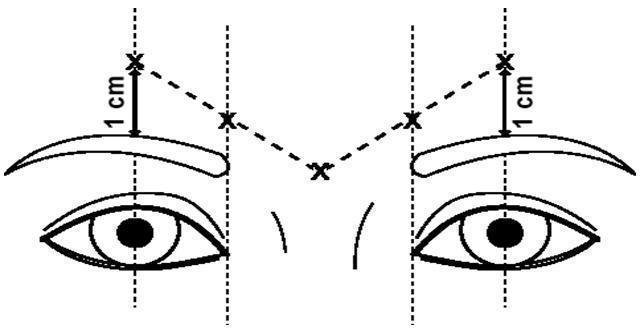
The dose of DYSPORT for the treatment of glabellar lines in adults is a total of 50 Units given intramuscularly in five equal aliquots of 10 Units each to achieve clinical effect (see Figure 1).
The clinical effect of DYSPORT may last up to four months. Repeat dosing in clinical studies demonstrated continued efficacy with up to four repeated administrations. It should be administered no more frequently than every three months. When used for re-treatment, DYSPORT should be reconstituted and injected using the same techniques as the initial treatment.
Injection Technique
Glabellar facial lines arise from the activity of the lateral corrugator and vertical procerus muscles. These can be readily identified by palpating the tensed muscle mass while having the patient frown. The corrugator depresses the skin creating a "furrowed" vertical line surrounded by tensed muscle (i.e., frown lines). The location, size, and use of the muscles vary markedly among individuals. Physicians administering DYSPORT must understand the relevant neuromuscular and/or orbital anatomy of the area involved and any alterations to the anatomy due to prior surgical procedures.
Risk of ptosis can be mitigated by careful examination of the upper lid for separation or weakness of the levator palpebrae muscle (true ptosis), identification of lash ptosis, and evaluation of the range of lid excursion while manually depressing the frontalis to assess compensation.
In order to reduce the complication of ptosis, the following steps should be taken:
- Avoid injection near the levator palpebrae superioris, particularly in patients with larger brow depressor complexes.
- Medial corrugator injections should be placed at least 1 centimeter above the bony supraorbital ridge.
- Ensure the injected volume/dose is accurate and where feasible kept to a minimum.
- Do not inject toxin closer than 1 centimeter above the central eyebrow.
To inject DYSPORT, advance the needle through the skin into the underlying muscle while applying finger pressure on the superior medial orbital rim. Inject patients with a total of 50 Units in five equally divided aliquots. Using an appropriately sized needle, inject 10 Units of DYSPORT into each of five sites, two in each corrugator muscle, and one in the procerus muscle (see Figure 1).
Figure 1
Dosing in Spasticity in Adults
Dosing in initial and subsequent treatment sessions should be tailored to the individual based on the size, number and location of muscles involved, severity of spasticity, the presence of local muscle weakness, the patient's response to previous treatment, and/or adverse reaction history with botulinum toxins.
No more than 1 mL should generally be administered at any single injection site. The maximum recommended total dose (upper and lower limb combined) of DYSPORT for the treatment of spasticity in adults is 1500 Units.
Although actual location of the injection sites can be determined by palpation, the use of injection guiding technique (e.g., electromyography, electrical stimulation, or ultrasound) is recommended to target the injection sites.
Upper Limb Spasticity
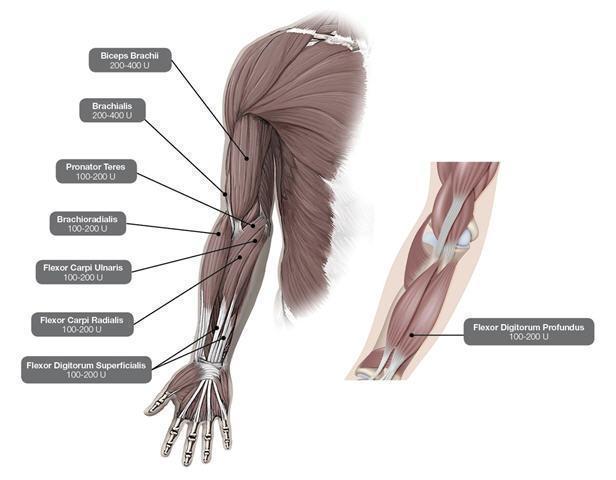
In the clinical trial that assessed the efficacy and safety of DYSPORT for treatment of upper limb spasticity in adults [ see Clinical Studies (14.3)], doses of 500 Units and 1000 Units were divided among selected muscles at a given treatment session (see Table 3and Figure 2).
| Muscles Injected | Recommended Dose DYSPORT |
Recommended Number of Injection(s) per Muscle |
|
Flexor carpi radialis (FCR) Flexor carpi ulnaris (FCU) |
100 Units to 200 Units 100 Units to 200 Units |
1 to 2 1 to 2 |
|
Flexor digitorum profundus (FDP) Flexor digitorum superficialis (FDS) |
100 Units to 200 Units 100 Units to 200 Units |
1 to 2 1 to 2 |
|
Brachialis Brachioradialis Biceps Brachii (BB) Pronator Teres |
200 Units to 400 Units 100 Units to 200 Units 200 Units to 400 Units 100 Units to 200 Units |
1 to 2 1 to 2 1 to 2 1 |
Figure 2: Muscles for Injection for Upper Limb Spasticity in Adults
Repeat DYSPORT treatment should be administered when the effect of a previous injection has diminished, but no sooner than 12 weeks after the previous injection. A majority of patients in clinical studies were retreated between 12-16 weeks; however some patients had a longer duration of response (i.e. 20 weeks). The degree and pattern of muscle spasticity at the time of re-injection may necessitate alterations in the dose of DYSPORT and muscles to be injected. Clinical improvement may be expected one week after administration of DYSPORT.
Lower Limb Spasticity
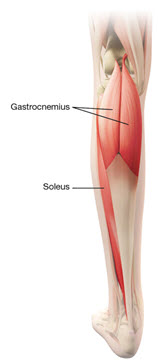
In the clinical trial that assessed the efficacy and safety of DYSPORT for treatment of lower limb spasticity in adults [ see Clinical Studies (14.3)], doses of 1000 Units and 1500 Units were divided among selected muscles at a given treatment session (see Table 4 and Figure 3).
| Muscles Injected | Recommended DYSPORT Dose | Recommended Number of Injection Sites per Muscle |
| Distal Muscles | ||
| Gastrocnemius | ||
| Medial head | 100 Units to 150 Units | 1 |
| Lateral head | 100 Units to 150 Units | 1 |
| Soleus | 330 Units to 500 Units | 3 |
| Tibialis posterior | 200 Units to 300 Units | 2 |
| Flexor digitorum longus | 130 Units to 200 Units | 1 to 2 |
| Flexor halluces longus | 70 Units to 200 Units | 1 |
Figure 3: Muscle for Injection for Lower Limb Spasticity in Adults

Repeat DYSPORT treatment should be administered when the effect of a previous injection has diminished, but no sooner than 12 weeks after the previous injection. A majority of patients in clinical studies were retreated between 12-16 weeks. The degree and pattern of muscle spasticity at the time of re-injection may necessitate alterations in the dose of DYSPORT and muscles to be injected.
Dosing in Spasticity in Pediatric Patients
DYSPORT dosing for spasticity in pediatric patients is based on Units per kilogram of body weight. To calculate the total units of DYSPORT required for treatment of one limb, select the dose of DYSPORT in Units/kg and the body weight (kg) of the patient (see Tables 5 and 6). Dosing in initial and sequential treatment sessions should be tailored to the individual patient based on the size, number and location of muscles involved, severity of spasticity, the presence of local muscle weakness, the patient’s response to previous treatment, and/or adverse reaction history with botulinum toxins.
No more than 0.5 mL should generally be administered at any single injection site. The maximum recommended total dose of DYSPORT in a single treatment session for spasticity in pediatric patients 2 years or older is 30 Units/kg or 1000 Units in a 3-month interval.
Although actual location of the injection sites can be determined by palpation, the use of injection guiding technique (e.g., electromyography or electrical stimulation, or ultrasound) is recommended to target the injection sites.
Upper Limb Spasticity in Pediatric Patients 2 Years of Age and Older
In the clinical trial that assessed the efficacy and safety of DYSPORT for treatment of upper limb spasticity in pediatric patients 2 years of age or older with a weight of at least 10 kg [ see Clinical Studies (14.4)], doses of 8 Units/kg or 16 Units/kg were divided among selected muscles of the target upper limb at a given treatment session (see Table 5 and Figure 4).
Table 5 describes the recommended Units/kg dose of DYSPORT per muscle. The maximum recommended total dose of DYSPORT administered for treatment of upper limb spasticity must not exceed 16 Units/kg or 640 Units, whichever is lower.
| Muscle | Recommended Dose Range per muscle per upper limb (units/kg Body Weight) |
Number of injection sites per muscle |
| Brachialis | 3 Units/kg to 6 Units/kg | Up to 2 |
| Brachioradialis | 1.5 Units/kg to 3 Units/kg | 1 |
| Biceps brachii | 3 Units/kg to 6 Units/kg | Up to 2 |
| Pronator teres | 1 Units/kg to 2 Units/kg | 1 |
| Prontator quadratus | 0.5 Unit/kg to 1 Units/kg | 1 |
| Flexor carpi radialis (FCR) | 2 Units/kg to 4 Units/kg | Up to 2 |
| Flexor carpi ulnaris (FCU) | 1.5 Units/kg to 3 Units/kg | 1 |
| Flexor digitorum profundus (FDP) | 1 Units/kg to 2 Units/kg | 1 |
| Flexor digitorum superficialis (FDS) | 1.5 Units/kg to 3 Units/kg | Up to 4 |
| Total dose | 8 Units/kg to 16 Units/kg in upper limbs (and not exceeding 640 Units) | |
Figure 4: Muscles for Injection for Upper Limb Spasticity in Pediatric Patients
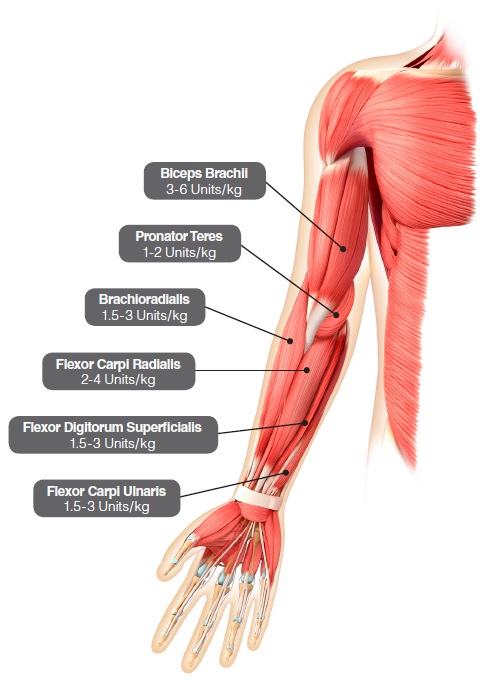

Repeat DYSPORT treatment should be administered when the effect of a previous injection has diminished but no sooner than 16 weeks after the previous injection. A majority of patients in the clinical study were retreated between 16-28 weeks; however, some patients had a longer duration of response (i.e., 34 weeks or more). The degree and pattern of muscle spasticity at the time of re-injection may necessitate alterations in the dose of DYSPORT and muscles to be injected.
Lower Limb Spasticity in Pediatric Patients 2 Years of Age and Older
In the clinical trial that assessed the efficacy and safety of DYSPORT for treatment of lower limb spasticity in pediatric patients 2 years of age or older, doses of 10 Units/kg to 15 Units/kg were divided among selected muscles of the target lower limb at a given treatment session (see Table 6 and Figure 5).
Table 6 describes the recommended Units/kg dose of DYSPORT per muscle of the Gastrocnemius-Soleus Complex (GSC). The recommended total DYSPORT dose per treatment session is 10 Units/kg to 15 Units/kg for unilateral lower limb injections or 20 Units/kg to 30 Units/kg for bilateral lower limb injections. However, the total dose of DYSPORT administered in a 3-month interval must not exceed 15 Units/kg for unilateral lower limb injections, 30 Units/kg for bilateral lower limb injections, or 1000 units, whichever is lower. The total dose administered should be divided between the affected spastic muscles of the lower limb(s). When possible, the dose should be distributed across more than 1 injection site in any single muscle (see Table 6).
|
||
| Muscle Injected | Recommended DYSPORT Dose Range per muscle per leg (Units/kg Body Weight) |
Recommended number of injections per muscle |
| Gastrocnemius | 6 Units/kg to 9 Units/kg * | Up to 4 |
| Soleus | 4 Units/kg to 6 Units/kg * | Up to 2 |
| Total | 10 Units/kg to 15 Units/kg divided across both muscles | Up to 6 |
Figure 5: Muscles for Injection for Lower Limb Spasticityin Pediatric Patients
Although actual location of the injection sites can be determined by palpation, the use of injection guiding technique (e.g. electromyography or electrical stimulation or ultrasound) is recommended to target the injection sites.
Repeat DYSPORT treatment should be administered when the effect of a previous injection has diminished but no sooner than 12 weeks after the previous injection. A majority of patients in the clinical studies were retreated between 16-22 weeks, however; some had a longer duration of response. The degree and pattern of muscle spasticity and overall clinical benefit at the time of re-injection may necessitate alterations in the dose of DYSPORT and muscles to be injected.
The safety and effectiveness of DYSPORT injected into proximal muscles of the lower limb for the treatment of spasticity in pediatric patients has not been established.
Frequently asked questions
More about Dysport (abobotulinumtoxinA)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (9)
- Side effects
- During pregnancy
- FDA approval history
- Drug class: skeletal muscle relaxants
- Breastfeeding
- En español
Patient resources
Professional resources
Related treatment guides
See also:
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.