Abilify Asimtufii Dosage
Generic name: Aripiprazole 720mg in 2.4mL
Dosage form: injection, suspension, extended release
Drug class: Atypical antipsychotics
Medically reviewed by Drugs.com. Last updated on Apr 4, 2025.
Important Administration Information
For patients who have never taken aripiprazole, establish tolerability with oral aripiprazole prior to initiating treatment with ABILIFY ASIMTUFII. Due to the half-life of oral aripiprazole (i.e., 75 hours and 94 hours for aripiprazole and dehydro-aripiprazole, respectively), it may take up to 2 weeks to fully assess tolerability.
ABILIFY ASIMTUFII must be administered as an intramuscular gluteal injection by a healthcare professional. Do not administer by any other route.
For detailed preparation and administration instructions,.
Recommended Dosage for ABILIFY ASIMTUFII
The recommended dosage of ABILIFY ASIMTUFII is 960 mg, administered once every 2 months (56 days after previous injection).
Patients Receiving Oral Antipsychotics
There are two ways to initiate treatment with ABILIFY ASIMTUFII in patients receiving oral antipsychotics:
1-day initiation:
- Administer one intramuscular injection of ABILIFY ASIMTUFII 960 mg in the gluteal muscle, one injection of Abilify Maintena 400 mg in a separate gluteal or deltoid muscle, and one dose of oral aripiprazole 20 mg, on the first day of treatment with ABILIFY ASIMTUFII.
- Do not administer both injections into the same muscle.
14-day initiation:
- Administer one intramuscular injection of ABILIFY ASIMTUFII 960 mg in the gluteal muscle and continue treatment with oral aripiprazole (10 mg to 20 mg) for 14 consecutive days.
- For patients already stable on another oral antipsychotic (and known to tolerate aripiprazole), administer one intramuscular injection of ABILIFY ASIMTUFII 960 mg in the gluteal muscle and continue treatment with the oral antipsychotic for 14 consecutive days.
Patients Receiving Abilify Maintena
For patients receiving Abilify Maintena 400 mg (once monthly dosing), administer ABILIFY ASIMTUFII 960 mg (once every 2 month dosing) in place of the next scheduled injection of the Abilify Maintena. The first ABILIFY ASIMTUFII injection may be administered in place of the second, or later injection of Abilify Maintena.
If there are adverse reactions with the ABILIFY ASIMTUFII 960 mg dosage, the dosage may be reduced to 720 mg once every 2 months.
Patients may be given the ABILIFY ASIMTUFII injection up to 2 weeks before or 2 weeks after the 2-month scheduled timepoint.
Missed Doses
If more than 8 weeks and less than 14 weeks have elapsed since the last injection, administer the next dose of ABILIFY ASIMTUFII as soon as possible. The once every 2 month schedule should be resumed.
If more than 14 weeks have elapsed since the last injection, restart treatment with either 1-day initiation or 14-day initiation with ABILIFY ASIMTUFII.
Dosage Modifications for Cytochrome P450 Considerations
Dosage adjustments for patients who are CYP2D6 poor metabolizers and/or in patients taking concomitant strong CYP3A4 inhibitors or CYP2D6 inhibitors for more than 14 days are described in Table 1.
If the CYP3A4 inhibitor or CYP2D6 inhibitor is withdrawn, the dosage of ABILIFY ASIMTUFII may need to be increased to the previous dose.
Dosage adjustments are not recommended for patients with concomitant use of CYP3A4 inhibitors, CYP2D6 inhibitors or CYP3A4 inducers for less than 14 days.
| Factors | Dosage Recommendation |
|---|---|
|
|
| CYP2D6 Poor Metabolizers | |
| Known CYP2D6 Poor Metabolizers | 720 mg once every 2 months* |
| Known CYP2D6 Poor Metabolizers taking concomitant CYP3A4 inhibitors | Avoid use |
| Patients Taking 960 mg of ABILIFY ASIMTUFII | |
| Concomitant use of ABILIFY ASIMTUFII with Strong CYP2D6 inhibitors | 720 mg once every 2 months* |
| Concomitant use of ABILIFY ASIMTUFII with Strong CYP3A4 inhibitors | 720 mg once every 2 months* |
| Concomitant use of ABILIFY ASIMTUFII with Strong CYP2D6 and Strong CYP3A4 inhibitors | Avoid use |
| Concomitant use of ABILIFY ASIMTUFII with CYP3A4 inducers | Avoid use |
Preparation and Administration Instructions
- Read the complete instructions for preparation and administration below and consider referring to the separate Healthcare Provider "Instructions for Use" for additional preparation and administration considerations.
- To be prepared and administered by a healthcare professional only.
- For gluteal intramuscular injection only. Do not administer by any other route.
- Prior to administration, visually inspect ABILIFY ASIMTUFII pre-filled syringe for particulate matter and discoloration. The suspension should appear to be a uniform, homogeneous suspension that is opaque and milky-white in color. Do not use ABILIFY ASIMTUFII pre-filled syringe if the suspension is discolored, or particulate matter is present
- Each kit contains one sterile pre-filled syringe containing ABILIFY ASIMTUFII 720 mg or 960 mg and two safety needles:
- One sterile 1 ½ inch, 22 gauge needle (in black packaging)
- One sterile 2 inch, 21 gauge needle (in green packaging)
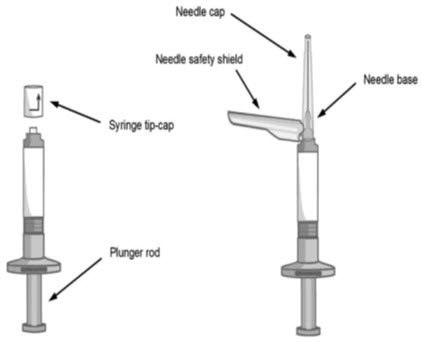
- Each kit contains one sterile pre-filled syringe containing ABILIFY ASIMTUFII 720 mg or 960 mg and two safety needles:
Preparation Prior to Administration
- Remove the ABILIFY ASIMTUFII pre-filled syringe from the package.
- Tap the syringe on your hand at least 10 (ten) times (Figure 1).
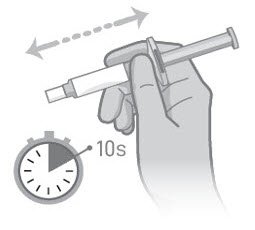
- After tapping, shake the syringe vigorously for at least 10 (ten) seconds, until the medication is uniform (Figure 2).
Figure 1 Figure 2 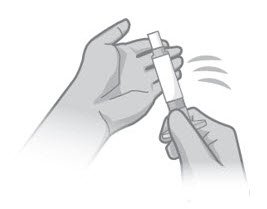
Select the appropriate needle
Needle selection is determined by patient body type.
For gluteal intramuscular administration only.
- For non-obese patients - 22-gauge, 1.5-inch (38 mm) safety needle with needle protection device (needle in black packaging)
- For obese patients - 21-gauge, 2-inch (51 mm) safety needle with needle protection device (needle in green packaging)
Attach the needle
- Twist and pull off the pre-filled syringe tip-cap (Figure 3).
- While holding the base of the needle, ensure the needle is firmly seated on the safety device with a push. Gently twist clockwise until SECURELY fitted (Figure 3).
Figure 3 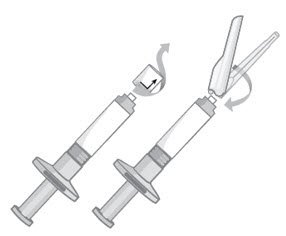
Expel Air
- When you are ready to administer the injection of ABILIFY ASIMTUFII, hold the pre-filled syringe upright and remove the needle-cap straight up (Figure 4). Do not twist the needle-cap, as this may loosen the needle from the syringe.
Figure 4 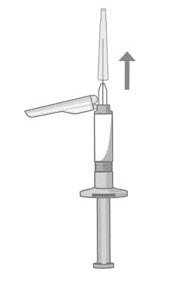
- Slowly advance the plunger rod upward to expel the air and until the suspension fills needle base (Figure 5).
Figure 5 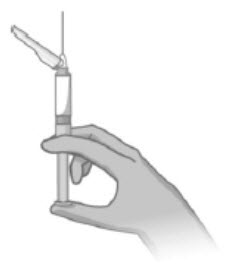
Inject the dose
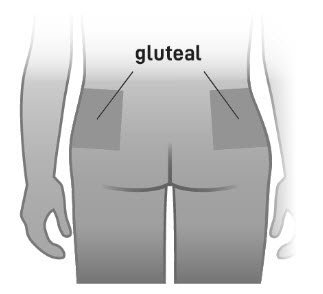
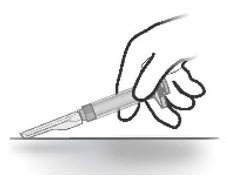
- Slowly inject the entire contents of the pre-filled syringe intramuscularly into the gluteal muscle of the patient (Figure 6).
Do not administer by any other route.
Do not massage the injection site.
| Figure 6 |
 |
Disposal Procedure
- After the injection, press the safety shield on a hard surface to cover and lock shield over the needle (Figures 7 and 8)
Figure 7 Figure 8 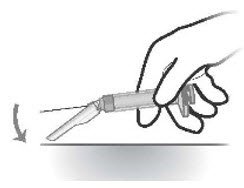
- Immediately discard used syringe and the unused needle in an approved sharps container.
- The unused needle should not be saved for future use.
Frequently asked questions
- Why should you take aripiprazole in the morning?
- Does Abilify cause weight gain?
- What is the difference between Abilify and Abilify Maintena?
- How does Abilify MyCite work?
- What drugs cause tardive dyskinesia?
More about Abilify Asimtufii (aripiprazole)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (1)
- Latest FDA alerts (5)
- Side effects
- During pregnancy
- FDA approval history
- Drug class: atypical antipsychotics
- Breastfeeding
- En español
Patient resources
Other brands
Abilify, Abilify Maintena, Aristada, Abilify MyCite, ... +3 more
Professional resources
Other brands
Abilify, Abilify Maintena, Aristada, Abilify MyCite, ... +2 more
Related treatment guides
See also:
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.









