Vtama Cream: Package Insert / Prescribing Info
Package insert / product label
Generic name: tapinarof
Dosage form: cream
Drug class: Topical antipsoriatics
Medically reviewed by Drugs.com. Last updated on Aug 3, 2025.
On This Page
Highlights of Prescribing Information
VTAMA (tapinarof) cream, for topical use
Initial U.S. Approval: 2022
Recent Major Changes
| Indications and Usage (1.2) | 12/2024 |
Indications and Usage for Vtama Cream
Vtama Cream Dosage and Administration
Contraindications
None. (4)
Adverse Reactions/Side Effects
- In plaque psoriasis, the most common adverse reactions (incidence ≥ 1%) were folliculitis, nasopharyngitis, contact dermatitis, headache, pruritus, and influenza. (6.1)
- In atopic dermatitis, the most common adverse reactions (incidence ≥ 1%) were upper respiratory tract infection, folliculitis, lower respiratory tract infection, headache, asthma, vomiting, ear infection, pain in extremity, and abdominal pain. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Organon LLC, a subsidiary of Organon & Co., at 1-844-674-3200 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 5/2025
Full Prescribing Information
1. Indications and Usage for Vtama Cream
2. Vtama Cream Dosage and Administration
Apply a thin layer of VTAMA cream to affected areas once daily.
Wash hands after application, unless VTAMA cream is for treatment of the hands.
VTAMA cream is not for oral, ophthalmic, or intravaginal use.
3. Dosage Forms and Strengths
Cream, 1%
Each gram of VTAMA cream contains 10 mg of tapinarof in a white to off-white cream.
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Plaque Psoriasis Clinical Trials
In two randomized, double-blind, multicenter, vehicle-controlled clinical trials (PSOARING 1 and PSOARING 2), 1025 adults with plaque psoriasis were treated with VTAMA cream or received vehicle cream once daily for up to 12 weeks.
Subjects ranged in age from 18 to 75 years, with an overall median age of 51 years. The majority of subjects were White (85%) and male (57%); and 85% identified as non-Hispanic or Latino.
Table 1 presents adverse reactions that occurred in at least 1% of subjects treated with VTAMA cream, and for which the rate exceeded the rate for vehicle.
| Adverse Reaction | VTAMA cream N=683 n (%) | Vehicle cream N=342 n (%) |
|---|---|---|
|
||
| Folliculitis* | 140 (20) | 3 (1) |
| Nasopharyngitis† | 73 (11) | 31 (9) |
| Contact dermatitis‡ | 45 (7) | 2 (1) |
| Headache§ | 26 (4) | 5 (1) |
| Pruritus¶ | 20 (3) | 2 (1) |
| Influenza# | 14 (2) | 2 (1) |
Two (0.3%) subjects using VTAMA cream developed urticaria. Adverse reactions leading to treatment discontinuation in >1% of subjects who received VTAMA cream were contact dermatitis (2.9%) and folliculitis (2.8%).
In an open label safety trial (PSOARING 3), 763 subjects were treated for up to an additional 40 weeks after completing PSOARING 1 or PSOARING 2. In addition to the adverse reactions reported in the 12-week PSOARING 1 and PSOARING 2 clinical trials, the following adverse reactions were reported: urticaria (1.0%) and drug eruption (0.7%).
Atopic Dermatitis Clinical Trials
In two randomized, double-blind, multicenter, vehicle-controlled clinical trials (ADORING 1 and ADORING 2), 811 adult and pediatric subjects 2 years of age and older with atopic dermatitis were treated with VTAMA cream or received vehicle cream once daily for up to 8 weeks.
Subjects ranged in age from 2 to 81 years, with an overall median age of 11 years. The majority (51%) of subjects were White, 31% were Black, 12% were Asian; 54% were female; and 78% of subjects identified as non-Hispanic or Latino.
Table 2 presents adverse reactions that occurred in at least 1% of subjects treated with VTAMA cream, and for which the rate exceeded the rate for vehicle.
| Adverse Reaction | VTAMA cream N=541 n (%) | Vehicle cream N=270 n (%) |
|---|---|---|
|
||
| Upper respiratory tract infection* | 66 (12) | 15 (6) |
| Folliculitis† | 51 (9) | 3 (1) |
| Lower respiratory tract infection‡ | 25 (5) | 6 (2) |
| Headache | 23 (4) | 3 (1) |
| Asthma§ | 12 (2) | 1 (0) |
| Vomiting | 10 (2) | 2 (1) |
| Ear infection¶ | 10 (2) | 1 (0) |
| Pain in extremity# | 9 (2) | 1 (0) |
| Abdominal painÞ | 6 (1) | 0 (0) |
Application site reactions were reported in 19 (3.5%) subjects treated with VTAMA cream and 9 (3.3%) subjects receiving vehicle.
The adverse reactions observed in pediatric subjects 2 years of age and older were generally consistent with those observed in adults with atopic dermatitis. Adverse reactions occurring more frequently in pediatric subjects compared to adults were upper respiratory tract infection (16.3% in subjects ages 2-6 years of age and 11.2% in subjects ages 7-17 years of age vs. 9.5% in subjects 18 years and older) and ear infection (5.7% in subjects ages 2-6 years of age and 1.4% in subjects ages 7-11 years of age vs. 0% in subjects 12 years and older).
In an open label safety trial (ADORING 3), 728 subjects (124 adult and 604 pediatric subjects 2 years of age and older) were treated for up to 48 weeks. This included 624 subjects completing either ADORING 1 or ADORING 2, 28 subjects completing the maximal usage trial, and 76 subjects treated only in ADORING 3. The safety profile with long term use was generally consistent with the safety profile observed at Week 8.
Related/similar drugs
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
The available data on VTAMA cream use in pregnant women are insufficient to evaluate for a drug-associated risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes. In animal reproduction studies, subcutaneous administration of tapinarof to pregnant rats and rabbits during the period of organogenesis resulted in no significant adverse effects at doses 264 and 16 times, respectively, the maximum recommended human dose (MRHD) (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of major birth defects, loss, and other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Animal Data
In an embryofetal development study in rats, tapinarof was administered by subcutaneous injection to pregnant animals at doses of 1.2, 6.9 and 34 mg/kg/day during the period of organogenesis. Tapinarof was not associated with embryofetal lethality or fetal malformations. Tapinarof increased the incidence of skeletal variations (incomplete ossification of nasal bones) at the dose of 34 mg/kg/day (264 times the MRHD based on AUC comparisons).
In an embryofetal development study in rabbits, tapinarof was administered by subcutaneous injection to pregnant animals twice daily at doses of 0.3, 1, and 3 mg/kg/day during the period of organogenesis. Maternal toxicity as evidenced by decreased maternal body weight gain and associated increased post-implantation loss (embryolethality) was observed at 3 mg/kg/day. In addition, fetal skeletal variations were observed at 3 mg/kg/day. Tapinarof was not associated with embryofetal lethality or fetal malformations at doses up to 1 mg/kg/day (16 times the MRHD based on AUC comparison) or fetal malformations at doses up to 3 mg/kg/day (30 times the MRHD based on AUC comparison).
In a second embryofetal development study in rabbits, tapinarof was administered by continuous subcutaneous infusion to pregnant animals at doses of 1, 2 and 3 mg/kg/day during the period of organogenesis. Tapinarof was not associated with embryofetal lethality or fetal malformations at doses up to 3 mg/kg/day (20 times the MRHD based on AUC comparison).
In a prenatal and postnatal development study, tapinarof was administered by subcutaneous injection to pregnant rats at doses of 1, 6 and 30 mg/kg/day beginning on gestation day 6 through lactation day 20. Maternal toxicity associated with decreases in body weight gain and food consumption was noted at 30 mg/kg/day (264 times the MRHD based on AUC comparisons). Tapinarof decreased fetal survival and viability that resulted in reduced litter sizes and decreased fetal weights at doses greater than or equal to 6 mg/kg/day (44 times the MRHD based on AUC comparisons). No tapinarof-related effects on fetal survival and viability were noted at a dose of 1 mg/kg/day (6 times the MRHD based on AUC comparisons). No tapinarof-related effects on postnatal development, neurobehavioral or reproductive performance of offspring were noted at doses up to 30 mg/kg/day (264 times the MRHD based on AUC comparison).
8.2 Lactation
Risk Summary
No data are available regarding the presence of tapinarof in human milk or the effects of tapinarof on the breastfed infant, or on milk production. Tapinarof was detected in rat offspring following subcutaneous administration to pregnant female rats which suggests that tapinarof was transferred into the milk of lactating rats (see Data). When a drug is present in animal milk, it is likely that the drug will be present in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for VTAMA cream and any potential adverse effects on the breastfed infant from VTAMA cream or from the underlying maternal condition.
In a prenatal and postnatal development study, tapinarof was administered by subcutaneous injection to pregnant rats at doses of 1, 6, and 30 mg/kg/day from gestation day 6 through lactation day 20. Tapinarof was quantifiable in offspring plasma samples on postnatal day 10 at doses of 6 and 30 mg/kg/day, suggesting that tapinarof is present in animal milk.
8.4 Pediatric Use
Plaque Psoriasis
The safety and efficacy of VTAMA cream for the topical treatment of plaque psoriasis have not been established in pediatric patients.
Atopic Dermatitis
The safety and efficacy of VTAMA cream for the topical treatment of atopic dermatitis have been established in pediatric patients 2 years of age and older. Use of VTAMA cream for this indication is supported by evidence from two adequate and well-controlled trials (ADORING 1 and ADORING 2), which included 654 pediatric subjects 2 years of age and older (436 of whom received VTAMA cream) with moderate to severe atopic dermatitis. Adverse reactions occurring more frequently in pediatric subjects compared to adults were upper respiratory tract infection and ear infection [see Adverse Reactions (6.1), Clinical Pharmacology (12.3) and Clinical Studies (14.2)].
The safety and efficacy of VTAMA cream for the topical treatment of atopic dermatitis have not been established in pediatric patients younger than 2 years of age.
Juvenile Animal Toxicity Data
In a juvenile animal toxicity study, tapinarof was administered by subcutaneous injection to juvenile rats at doses of 1, 10 and 20 mg/kg/day from postnatal day (PND) 7 to 21 and at doses of 1.5, 15, and 30 mg/kg/day from PND 22 to 77. The dose escalation conducted at PND 22 was implemented to maintain consistent systemic exposure across the duration of the dosing period. Renal pelvic dilatation was observed at doses greater than or equal to 15 mg/kg/day (165 times the MRHD based on AUC comparisons). No adverse effects in juvenile animals were noted at 1.5 mg/kg/day (11 times the MRHD based on AUC comparisons).
8.5 Geriatric Use
Plaque Psoriasis
Of the 683 subjects exposed to VTAMA cream in the PSOARING 1 or PSOARING 2 clinical trials, 99 (14.5%) were 65 years of age and older, including 8 (1.2%) subjects who were 75 years of age and older. No overall differences in efficacy, safety, or tolerability of VTAMA cream were observed between subjects 65 years of age and older and younger adult subjects.
Atopic Dermatitis
Of the 541 subjects exposed to VTAMA cream in the ADORING 1 or ADORING 2 clinical trials, 20 (3.7%) were 65 years of age and older, including 4 (0.7%) subjects who were 75 years of age and older. No overall differences in efficacy, safety, or tolerability were observed between subjects 65 years of age and older and younger adult subjects.
11. Vtama Cream Description
VTAMA (tapinarof) cream contains tapinarof as the active ingredient. Tapinarof is an aryl hydrocarbon receptor agonist.
Tapinarof is a white to pale brown powder. Chemically, tapinarof is 3, 5-dihydroxy-4-isopropyl-trans-stilbene, also known as (E)-2-isopropyl-5-styrylbenzene-1,3-diol, with the empirical formula C17H18O2, a molecular weight of 254.32, and the following structural formula.
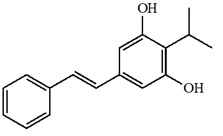 |
Each gram of VTAMA cream for topical use contains 10 mg of tapinarof in a white to off-white cream. VTAMA cream also contains the following inactive ingredients: benzoic acid, butylated hydroxytoluene, citric acid monohydrate, diethylene glycol monoethyl ether, edetate disodium, emulsifying wax, medium-chain triglycerides, polyoxyl 2 stearyl ether, polyoxyl 20 stearyl ether, polysorbate 80, propylene glycol, purified water, and sodium citrate dihydrate.
12. Vtama Cream - Clinical Pharmacology
12.1 Mechanism of Action
Tapinarof is an aryl hydrocarbon receptor (AhR) agonist. The specific mechanisms by which VTAMA cream exerts its therapeutic actions are unknown.
12.2 Pharmacodynamics
Pharmacodynamics of VTAMA cream are unknown.
Cardiac Electrophysiology
At the approved recommended dosage, VTAMA cream does not prolong the QTc interval to any clinically relevant extent.
12.3 Pharmacokinetics
The plasma concentrations of tapinarof were higher in pediatric subjects 2 years of age and older with atopic dermatitis when compared to adult subjects with plaque psoriasis.
Absorption
Adults with Plaque Psoriasis
No accumulation was observed with repeat topical application. Plasma concentration of tapinarof was below the quantifiable limits (BQL) of the assay (lower limit of quantification was 50 pg/mL) in 68% of the PK samples. On Day 1, mean ± SD values of Cmax and AUC0-last were 0.90 ± 1.4 ng/mL and 4.1 ± 6.3 ng.h/mL, respectively, following a mean daily dose of 5.23 g applied to a mean body surface area (BSA) involvement of 27.2% (range 21 to 46%) in 21 subjects with moderate to severe plaque psoriasis. On Day 29, the mean ± SD Cmax and AUC0-last were 0.12 ± 0.15 ng/mL and 0.61 ± 0.65 ng.h/mL, respectively.
Pediatric Subjects with Atopic Dermatitis
The PK of VTAMA cream were investigated in 36 pediatric subjects 2 years of age and older with moderate to severe atopic dermatitis and a mean ± SD BSA involvement of 43% ± 15% (range 26% to 90%). In this study, subjects applied approximately 3.2 g/day for 27 days.
Plasma concentrations were BQL in 25% of PK samples on Day 1 and 76% of PK samples on Day 28. On Day 1, the mean ± SD Cmax and AUC0-last were 2.4 ± 3.9 ng/mL and 4.7 ± 5.6 ng.h/mL, respectively in the overall pediatric population with atopic dermatitis. The mean ± SD Cmax and AUC0-last on Day 1 in pediatric subjects with atopic dermatitis stratified by age is shown in Table 3.
| PK Parameter (Mean ± SD) | 2 – 6 Years | 7 – 11 Years | 12 – 17 Years |
|---|---|---|---|
| Cꚍ: concentration at the end of 24 h dosing interval on Day 28. | |||
| Day 1 | |||
| Cmax (ng/mL) | 3.8 ± 5.87 | 2.2 ± 2.5 | 1.3 ± 1.8 |
| AUC0-t (ng.h/mL) | 5.5 ± 6.2 | 5.2 ± 5.9 | 3.3 ± 4.8 |
| Day 28 | |||
| Cꚍ (ng/mL) | 0.046 ± 0.107 | 0.089 ± 0.130 | 0.125 ± 0.413 |
Distribution
Human plasma protein binding of tapinarof is approximately 99% in vitro.
Elimination
Metabolism
Tapinarof is metabolized in the liver by multiple pathways including oxidation, glucuronidation, and sulfation in vitro.
Drug Interaction Studies
In Vitro Studies
Cytochrome P450 (CYP) Enzymes: Tapinarof is not an inhibitor of CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6 or CYP3A4/5. Tapinarof is not an inducer of CYP1A2, CYP2B6 or CYP3A4.
Transporter Systems: Tapinarof is not an inhibitor of BCRP, MATE1, MATE-2K, OAT1, OAT3, OATP1B1, OATP1B3, OCT1, OCT2, or P-gp. Tapinarof is not a substrate for BCRP, OATP1B1, OATP1B3, or P-gp.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term carcinogenicity studies were conducted in mice (daily topical administration at doses of 0.5, 1.5 and 3% tapinarof cream) and in rats (subcutaneous administration at doses of 0.1, 0.3, and 1 mg/kg/day tapinarof). No drug-related neoplasms were noted in mice after 98 (females) to 102 (males) weeks of daily topical administration at doses up to 3% tapinarof cream (44 times the MRHD based on AUC comparisons). No drug-related neoplasms were noted in female rats after 83 weeks of daily subcutaneous administration at doses up to 1 mg/kg/day tapinarof (9 times the MRHD based on AUC comparisons).
Tapinarof revealed no evidence of mutagenicity or clastogenicity in an Ames assay, an in vitro mammalian chromosomal aberration assay, an in vitro mouse lymphoma assay and two in vivo micronucleus-assays in mice and rats.
Tapinarof did not impair female fertility at subcutaneous doses up to 30 mg/kg/day (264 times the MRHD based on AUC comparisons).
14. Clinical Studies
14.1 Plaque Psoriasis
Two multicenter, randomized, double-blind, vehicle-controlled trials were conducted to evaluate the safety and efficacy of VTAMA cream for the treatment of adults with plaque psoriasis (PSOARING 1 [NCT03956355] and PSOARING 2 [NCT03983980]). These trials were conducted in a total of 1025 subjects randomized 2:1 to VTAMA cream or vehicle cream applied once daily for 12 weeks to any lesion regardless of anatomic location.
Baseline disease severity was graded using the 5-point Physician’s Global Assessment (PGA). The majority of subjects had “Moderate” disease (82%), while 10% had “Mild” disease, and 8% had “Severe” disease at baseline. The extent of disease involvement assessed by mean body surface area (BSA), excluding the scalp, palms, and soles, was 8% (range 3 to 20%). Subjects ranged in age from 18 to 75 years, with a median age of 51 years. Overall, 57% of the subjects were male and 85% were White.
The primary efficacy endpoint in both studies was the proportion of subjects who achieved treatment success, defined as a PGA score of “Clear” (0) or “Almost Clear” (1) and at least a 2-grade improvement from baseline. Efficacy results from the two trials are summarized in Table 4.
| Clinical Response | PSOARING 1 | PSOARING 2 | ||
|---|---|---|---|---|
| VTAMA cream N=340 | Vehicle cream N=170 | VTAMA cream N=343 | Vehicle cream N=172 |
|
|
||||
| PGA Treatment Success * | 36% | 6% | 40% | 6% |
| Difference (95% CI) | 29% (22%, 36%) | 34% (27%, 41%) | ||
Following 12 weeks of treatment, 73 subjects randomized to VTAMA achieved complete disease clearance (PGA 0) and had VTAMA withdrawn. These subjects were followed for up to 40 additional weeks with a median time to first worsening (PGA ≥ 2 [“Mild”]) of 114 days (95% CI: 85, 142).
14.2 Atopic Dermatitis
Two multicenter, randomized, double-blind, vehicle-controlled trials were conducted to evaluate the safety and efficacy of VTAMA cream for the treatment of adult and pediatric subjects 2 years of age and older with atopic dermatitis (ADORING 1 [NCT05014568] and ADORING 2 [NCT05032859]). These trials were conducted in a total of 813 subjects (80% of subjects were 2 to 17 years of age) randomized 2:1 to VTAMA cream or vehicle cream applied once daily for 8 weeks to any lesion regardless of anatomic location.
Baseline disease severity was graded using the 5-point validated Investigator’s Global Assessment (vIGA-AD™). The majority of subjects had “Moderate” disease (87%), while 13% had “Severe” disease at baseline. The extent of disease involvement assessed by mean affected BSA, excluding the scalp, was 16.8% (range 5 to 44%). The mean baseline Eczema Area and Severity Index (EASI) score was 12.9 (range 4.4 to 36.0). The mean baseline Peak Pruritus Numeric Rating Scale (PP-NRS) score for subjects 12 years of age and older was 6.4 on a scale of 0-10. Subjects ranged in age from 2 to 81 years, with a median age of 11 years. Overall, 54% of the subjects were female, 51% were White, 31% were Black, and 12% were Asian. For ethnicity, 78% of subjects identified as non-Hispanic or Latino.
The primary efficacy endpoint in both trials was the proportion of subjects who achieved treatment success, defined as a vIGA-AD score of “Clear” (0) or “Almost Clear” (1) and at least a 2-grade improvement from baseline. Efficacy was also assessed using a ≥ 4-point improvement in PP-NRS score in subjects 12 years of age and older. Efficacy results from the two trials are summarized in Table 5.
| ADORING 1 | ADORING 2 | |||
|---|---|---|---|---|
| VTAMA cream | Vehicle cream | VTAMA cream | Vehicle cream | |
| Number of subjects randomized | 270 | 137 | 271 | 135 |
| vIGA-AD Treatment Success*,† Difference from Vehicle (95% CI) | 45% 32% (23%, 40%) | 14% | 46% 29% (19%, 38%) | 18% |
| Number of subjects ≥12 years of age with baseline PP-NRS score ≥4 | 103 | 54 | 126 | 64 |
| ≥ 4-point improvement in PP-NRS‡
Difference from Vehicle (95% CI) | 56% 22% (5%, 38%) | 34% | 53% 29% (15%, 43%) | 24% |
Of the 431 subjects randomized to VTAMA who completed 8 weeks of treatment in ADORING 1 and ADORING 2 and elected to continue follow-up, 51 subjects achieved complete disease clearance (vIGA-AD = 0) and had VTAMA withdrawn. These subjects were followed for up to 48 additional weeks with a median time to first worsening (vIGA-AD ≥ 2 [“Mild”]) of 57 days (95% CI: 30, 79).
16. How is Vtama Cream supplied
VTAMA (tapinarof) cream, 1% is a white to off-white cream. Each gram of VTAMA cream contains 10 mg of tapinarof. It is supplied in the following size:
60 g laminated tubes: NDC 81672-5051-1
Storage and Handling:
- -
- Store at 20°C to 25°C (68°F to 77°F) excursions permitted between 15°C and 30°C (59°F and 86°F) [See USP Controlled Room Temperature].
- -
- Do not freeze.
- -
- Protect from exposure to excessive heat.
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Administration Instructions [see Dosage and Administration (2)]
- Instruct patients to apply VTAMA cream once daily to affected skin lesions only and avoid unaffected areas of skin.
- Advise patients to wash hands after application unless VTAMA cream is for treatment of the hands.
- Advise patients that VTAMA cream is for external use only.
Dist. by: Organon LLC, a subsidiary of
ORGANON & Co.,
Jersey City, NJ 07302, USA
For patent information: www.organon.com/our-solutions/patent/
vIGA-AD is the trademark of Eli Lilly and Co.
© 2025 Organon group of companies. All rights reserved.
uspi-og0505-cr-2505r000
| PATIENT INFORMATION VTAMA® (Vee-TAM-uh) (tapinarof) cream, for topical use |
|
|---|---|
| This Patient Information has been approved by the U.S. Food and Drug Administration. | Revised: 5/2025 |
| Important information: VTAMA cream is for use on the skin (topical use) only. Do not use VTAMA cream in your eyes, mouth, or vagina. | |
| What is VTAMA cream?
VTAMA cream is a prescription medicine used on the skin (topical) to treat:
It is not known if VTAMA cream is safe and effective for the topical treatment of atopic dermatitis in children under 2 years of age. |
|
Before using VTAMA cream, tell your healthcare provider about all of your medical conditions, including if you:
|
|
How should I use VTAMA cream?
|
|
| What are the possible side effects of VTAMA cream? The most common side effects of VTAMA cream in people treated for plaque psoriasis include: |
|
|
|
| The most common side effects of VTAMA cream in people treated for atopic dermatitis include: | |
|
|
| VTAMA cream can also cause skin reactions at the treatment site. Tell your healthcare provider if you have itching, irritation, pain, redness, burning or changes in skin color at the treatment site. These are not all the possible side effects of VTAMA cream. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|
How should I store VTAMA cream?
|
|
| General information about the safe and effective use of VTAMA cream.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use VTAMA cream for a condition for which it was not prescribed. Do not give VTAMA cream to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about VTAMA cream that is written for health professionals. |
|
| What are the ingredients in VTAMA cream?
Active ingredient: tapinarof Inactive ingredients: benzoic acid, butylated hydroxytoluene, citric acid monohydrate, diethylene glycol monoethyl ether, edetate disodium, emulsifying wax, medium-chain triglycerides, polyoxyl 2 stearyl ether, polyoxyl 20 stearyl ether, polysorbate 80, propylene glycol, purified water, and sodium citrate dihydrate. Dist. by: Organon LLC, a subsidiary of ORGANON & Co., Jersey City, NJ 07302, USA For patent information: www.organon.com/our-solutions/patent/ © 2025 Organon group of companies. All rights reserved. usppi-og0505-cr-2505r000 For more information, go to www.VTAMA.com or call 1-844-674-3200. |
|
| VTAMA
tapinarof cream |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Organon LLC (117494753) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Glaxo Operations UK Ltd | 228472833 | MANUFACTURE(81672-5051) , PACK(81672-5051) , ANALYSIS(81672-5051) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bora Pharmaceutical Services Inc | 205556368 | MANUFACTURE(81672-5051) , PACK(81672-5051) , ANALYSIS(81672-5051) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Diteba Inc | 243296875 | ANALYSIS(81672-5051) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Thermo Fisher Scientific Cork Limited | 985745028 | API MANUFACTURE(81672-5051) , ANALYSIS(81672-5051) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Patheon Austria GmbH & CoKG | 300398976 | API MANUFACTURE(81672-5051) , ANALYSIS(81672-5051) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| PPD Development, L.P. | 838082055 | ANALYSIS(81672-5051) | |
More about Vtama (tapinarof topical)
- Compare alternatives
- Pricing & coupons
- Reviews (38)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- Drug class: topical antipsoriatics
- Breastfeeding
- En español



