Vazalore: Package Insert / Prescribing Info
Package insert / product label
Generic name: aspirin
Dosage form: capsule
Drug classes: Platelet aggregation inhibitors, Salicylates
Medically reviewed by Drugs.com. Last updated on Jan 9, 2025.
Indications and Usage for Vazalore
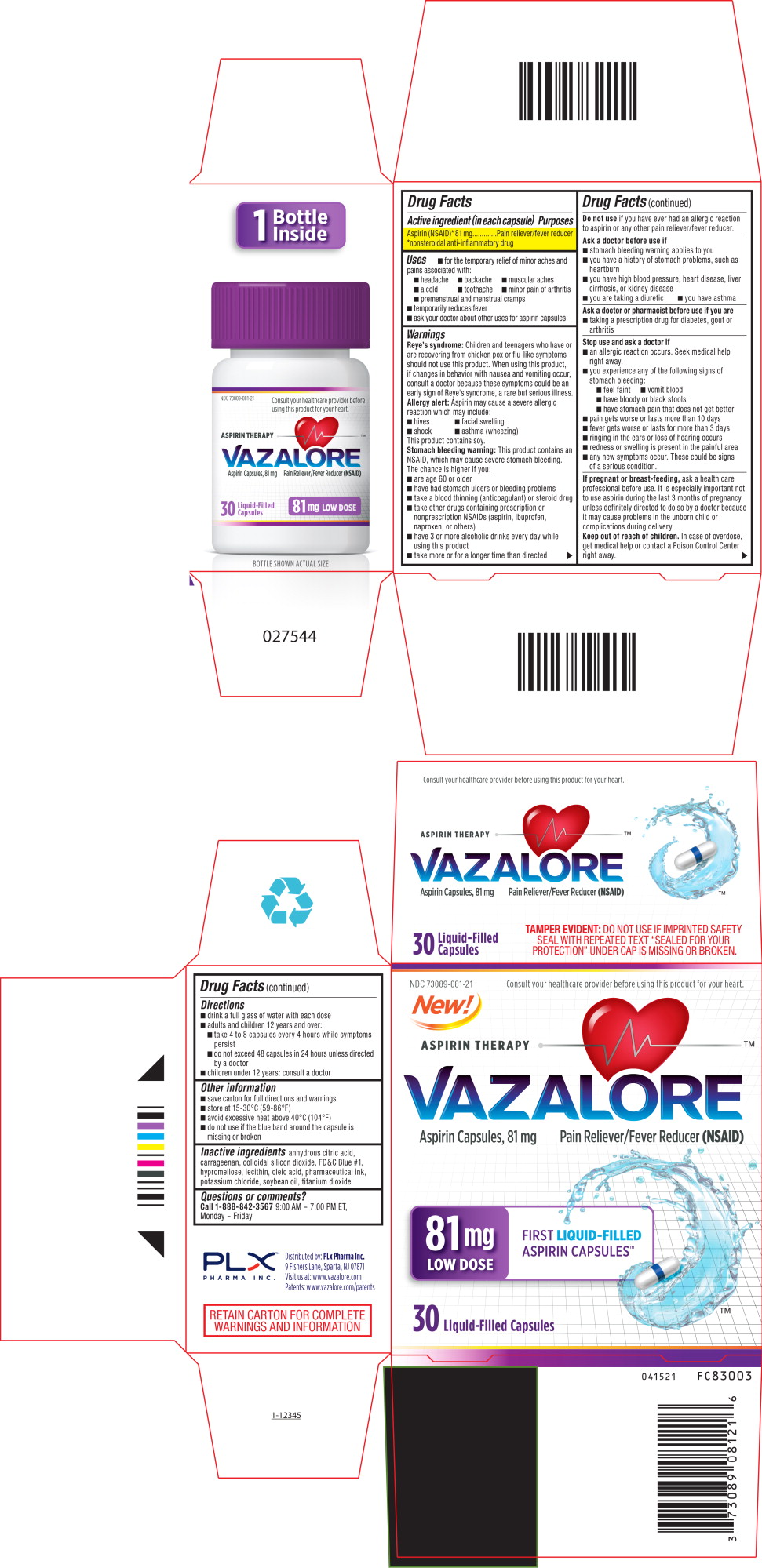
- For temporary relief of minor aches and pains associated with:
- headache
- Backache
- muscular aches
- a cold
- toothache
- minor pains of arthritis
- premenstrual and menstrual cramps
- temporarily redices fever
- n ask your doctor about other uses of aspirin capsules
Warnings
Warnings
Reye’s Syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye’s syndrome, a rare but serious illness.
Allergy Alert: Aspirin may cause a severe allergic reaction which may include
n hives n facial wseelling n shock n astham (wheezing)
This product contains soy
Stomach bleeding warning: This product contains an NSAID, which may cause bleeding. The chance is higher if you:
- Are age 60 or older
- Have had stomach ulcers or bleeding problems
- Take a blood thinning (anticoagulant) or steroid drug
- Take other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen, or others)
- Have 3 or more alcoholic drinks every day while using this product
- Take more or for a longer time than directed
Ask a doctor before use if:
- Stomach bleeding warning applies to you
- You have a history of stomach problems, such as heartburn
- You have high blood pressure, heart disease, liver cirrhosis, or kidney disease
- You are taking a diuretic
- You have asthma
Do not use If you have ever had an allergic reaction to aspirin or any other pain reliever / fever reducer
Ask a doctor or a pharmacist before you use if you are
- Taking a drug for diabetes, gout or arthritis
Stop use and ask a doctor
Stop use and ask a doctor if
- An allergic reaction occurs. Seek medical help right away
- You experience amu of the following signs of stomach bleeding
- Feel faint - vomit blood - have bloody or black stool - have stomach pain that does not get better
- Pain gets worse or lasts more than 10 days
- Fever gets worse or lasts more than 3 days
- Ringing in the ears or loss of hearing occurs
- Redness and swelling is present in the painful area
- Any new symptoms occur. These could be signs of serious conditions
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away
If pregnant or breast feeding, ask a healthcare professional before use. It is especially important not to use aspirin during the last 3 months of pregnancy unless definitely directed to do so by a doctor because it may cause problems in the unborn child or complications during delivery
Purposes:
Aspirin (NSAID)* 325mg __ Pain reliever / fever reducer
*Non steroidal Anti inflammatory Drug
Questions or Comments
Questions or comments?
Call 1-888-842-3569 9:00 AM – 7:00 PM ET, Monday – Friday
RETAIN CARTON FOR COMPLETE WARNINGS AND INFORMATION
Storage and Handling
Other Information
- Save carton for full directions and warnings
- Store at 15-30°C (59-86°F)
- Avoid excessive heat above 40°C (104°F)
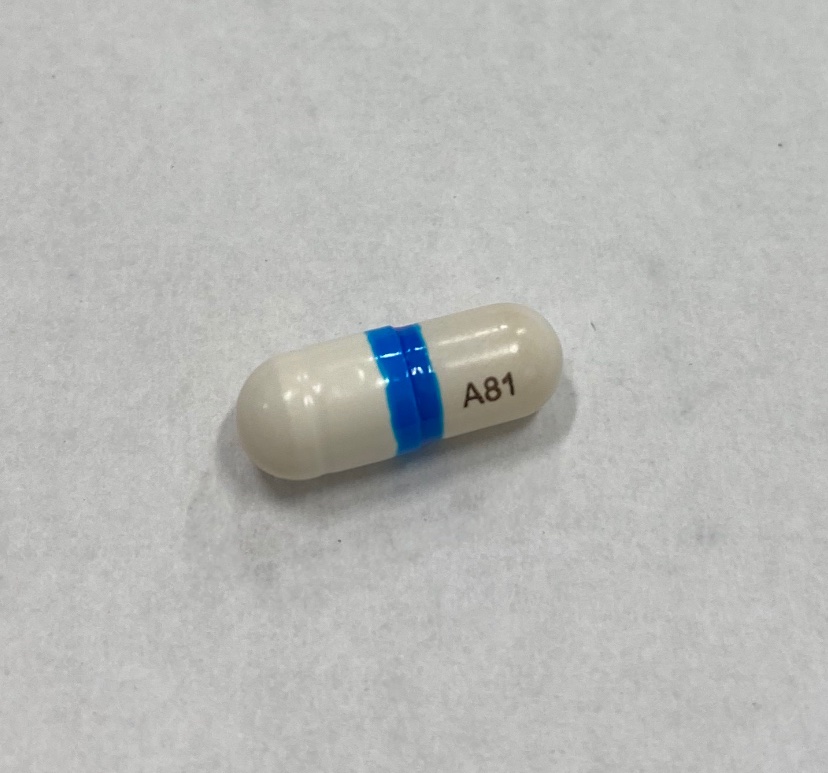
- Do not use if the blue band around the capsule is missing or broken
Inactive ingredients
Anhydrous citric acid, carrageenan, colloidal silicon dioxide, FD&C blue #1, Hypromellose, lecithin, oleic acid, pharmaceutical ink, potassium chloride, soybean oil, titanium dioxide
| VAZALORE
aspirin capsule |
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
 |
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| Labeler - PLx Pharma Inc (079325568) |
| Registrant - PLx Pharma Inc (079325568) |
Frequently asked questions
- What's the difference between aspirin and ibuprofen?
- Can You Take Tramadol with Acetaminophen, Ibuprofen, or Aspirin?
- What temperature is considered a fever?
- What cold medicine can you take with diabetes?
- Aspirin Overdose: Symptoms, Diagnosis, Emergency Treatment
- Does aspirin lower blood pressure?
- Which painkiller should you use?
- Can I give Aspirin to my dog or cat?
- Can you take ibuprofen with Excedrin Migraine?
More about Vazalore (aspirin)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (1)
- Drug images
- Latest FDA alerts (3)
- Side effects
- Dosage information
- During pregnancy
- Drug class: platelet aggregation inhibitors
- Breastfeeding
- En español