Clarinex-D 12 Hour: Package Insert / Prescribing Info
Package insert / product label
Generic name: desloratadine and pseudoephedrine sulfate
Dosage form: tablet, extended release
Drug class: Upper respiratory combinations
Medically reviewed by Drugs.com. Last updated on Dec 15, 2024.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Drug Interactions
- Use In Specific Populations
- Drug Abuse and Dependence
- Overdosage
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
CLARINEX-D® 12 HOUR Extended Release Tablets (desloratadine/pseudoephedrine sulfate) for oral use
Initial U.S. Approval: 2005
Indications and Usage for Clarinex-D 12 Hour
CLARINEX-D 12 HOUR is a combination product containing a histamine-1 (H1) receptor antagonist and an alpha adrenergic agonist indicated for:
- Relief of nasal and non-nasal symptoms of seasonal allergic rhinitis, including nasal congestion, in adults and adolescents 12 years of age and older. (1.1)
Clarinex-D 12 Hour Dosage and Administration
Dosage Forms and Strengths
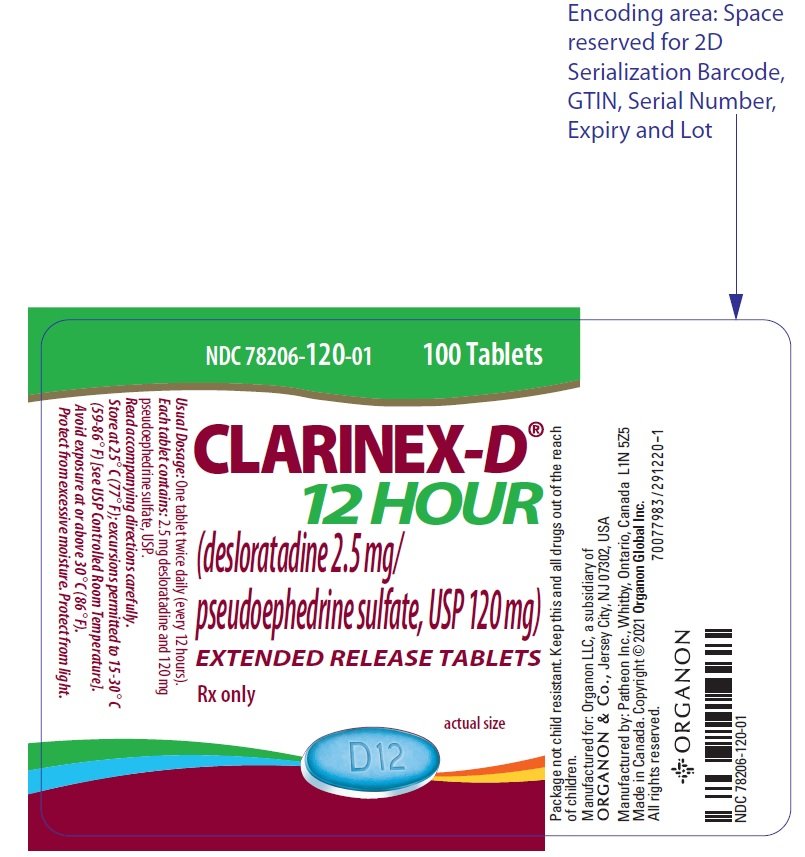
Desloratadine 2.5 mg/Pseudoephedrine sulfate 120 mg tablets. (3)
Contraindications
Warnings and Precautions
Adverse Reactions/Side Effects
- The most common adverse reactions (reported in ≥2% of patients) were insomnia, headache, mouth dry, fatigue, somnolence, pharyngitis, dizziness, nausea, and anorexia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Organon LLC, a subsidiary of Organon & Co., at 1-844-674-3200 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
Monoamine Oxidase (MAO) Inhibitors: Do not use. May potentiate the effect of pseudoephedrine on vascular system. (7.1)
Use In Specific Populations
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 6/2021
Full Prescribing Information
1. Indications and Usage for Clarinex-D 12 Hour
1.1 Seasonal Allergic Rhinitis
CLARINEX-D® 12 HOUR Extended Release Tablets is indicated for the relief of the nasal and non-nasal symptoms of seasonal allergic rhinitis, including nasal congestion, in adults and adolescents 12 years of age and older. CLARINEX-D 12 HOUR Extended Release Tablets should be administered when the antihistaminic properties of desloratadine and the nasal decongestant properties of pseudoephedrine are desired [see Clinical Pharmacology (12)].
2. Clarinex-D 12 Hour Dosage and Administration
Administer CLARINEX-D 12 HOUR Extended Release Tablet by the oral route only. Do not break, chew, or crush the tablet. Swallow the tablet whole.
2.1 Adults and Adolescents 12 years of Age and Over
The recommended dose of CLARINEX-D 12 HOUR Extended Release Tablets is 1 tablet twice a day, administered approximately 12 hours apart and with or without a meal. Higher doses or increased dosing frequency of CLARINEX-D 12 HOUR Extended Release Tablets have not demonstrated increased effectiveness. Do not exceed the recommended dose as desloratadine and pseudoephedrine, the active components of CLARINEX-D 12 HOUR Extended Release Tablets have been associated with adverse effects at higher doses [see Overdosage (10.1) and (10.2)].
3. Dosage Forms and Strengths
CLARINEX-D 12 HOUR Extended Release Tablets are oval shaped, blue and white bilayer tablets with "D12" embossed in the blue layer. Each tablet contains 2.5 mg desloratadine in the blue immediate-release layer and 120 mg of pseudoephedrine sulfate USP in the white extended-release layer.
4. Contraindications
CLARINEX-D 12 HOUR Extended Release Tablets are contraindicated in:
- Patients with hypersensitivity to any of its ingredients, or to loratadine [see Warnings and Precautions (5.4) and Adverse Reactions (6.2)]
- Patients with narrow-angle glaucoma
- Patients with urinary retention
- Patients receiving monoamine oxidase (MAO) inhibitor therapy or within fourteen (14) days of stopping such treatment [see Drug Interactions (7.1)]
- Patients with severe hypertension or severe coronary artery disease
5. Warnings and Precautions
5.1 Cardiovascular and Central Nervous System Effects
The pseudoephedrine sulfate contained in CLARINEX-D 12 HOUR Extended Release Tablets, like other sympathomimetic amines, can produce cardiovascular and central nervous system (CNS) effects in some patients such as insomnia, dizziness, weakness, tremor, or arrhythmias. In addition, central nervous system stimulation with convulsions or cardiovascular collapse with accompanying hypotension has been reported. Therefore, CLARINEX-D 12 HOUR Extended Release Tablets should be used with caution in patients with cardiovascular disorders, and should not be used in patients with severe hypertension or severe coronary artery disease.
5.2 Coexisting Conditions
CLARINEX-D 12 HOUR Extended Release Tablets contain pseudoephedrine sulfate, a sympathomimetic amine, and therefore should be used with caution in patients with diabetes and hyperthyroidism. Also use with caution in patients with prostatic hypertrophy or increased intraocular pressure, as urinary retention and narrow-angle glaucoma may occur [see Contraindications (4)].
5.3 Co-Administration with Monoamine Oxidase (MAO) Inhibitors
CLARINEX-D 12 HOUR Extended Release Tablets should not be used in patients receiving monoamine oxidase (MAO) inhibitor therapy or within fourteen (14) days of stopping such treatment as an increase in blood pressure or hypertensive crisis, may occur [see Contraindications (4) and Drug Interactions (7.1)].
5.4 Hypersensitivity Reactions
Hypersensitivity reactions including rash, pruritus, urticaria, edema, dyspnea, and anaphylaxis have been reported after administration of desloratadine a component of CLARINEX-D 12 HOUR Extended Release Tablets. If such a reaction occurs, therapy with CLARINEX-D 12 HOUR Extended Release Tablets should be stopped and alternative treatment should be considered [see Adverse Reactions (6.2)].
5.5 Renal Impairment
CLARINEX-D 12 HOUR Extended Release Tablets should generally be avoided in patients with renal impairment [see Clinical Pharmacology (12.3)].
5.6 Hepatic Impairment
CLARINEX-D 12 HOUR Extended Release Tablets should generally be avoided in patients with hepatic impairment [see Clinical Pharmacology (12.3)].
6. Adverse Reactions/Side Effects
The following adverse reactions are discussed in greater detail in other sections of the label:
- Cardiovascular and Central Nervous System effects [see Warnings and Precautions (5.1)]
- Increased intraocular pressure [see Warnings and Precautions (5.2)]
- Urinary retention in patients with prostatic hypertrophy [see Warnings and Precautions (5.2)]
- Hypersensitivity reactions [see Warnings and Precautions (5.4)]
- Severe Skin Reactions
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The safety data described below are from 2 clinical trials with CLARINEX-D 12 HOUR Extended Release Tablets that included 1248 patients with seasonal allergic rhinitis, of which 414 patients received CLARINEX-D 12 HOUR Extended Release Tablets twice daily for up to 2 weeks. The majority of patients were between 18 and <65 years of age with a mean age of 35.8 years and were predominantly women (64%). Patient ethnicity was 82% Caucasian, 9% Black, 6% Hispanic and 3% Asian/other ethnicity. The percentage of subjects receiving CLARINEX-D 12 HOUR Extended Release Tablets and who discontinued from the clinical trials because of an adverse event was 3.6%. Adverse reactions that were reported by ≥2% of subjects receiving CLARINEX-D 12 HOUR Extended Release Tablets are shown in Table 1.
| Adverse Reaction | CLARINEX-D 12 HOUR BID (N=414) | Desloratadine 5 mg QD (N=412) | Pseudoephedrine 120 mg BID (N=422) |
|---|---|---|---|
| Gastrointestinal Disorders | |||
| Mouth Dry | 8% | 2% | 8% |
| Nausea | 2% | 1% | 3% |
| General Disorders and Administration Site Conditions | |||
| Fatigue | 4% | 2% | 2% |
| Metabolism and Nutrition Disorders | |||
| Anorexia | 2% | 0% | 2% |
| Nervous System Disorders | |||
| Headache | 8% | 8% | 9% |
| Somnolence | 3% | 4% | 2% |
| Dizziness | 3% | 2% | 2% |
| Psychiatric Disorders | |||
| Insomnia | 10% | 3% | 13% |
| Respiratory, Thoracic, and Mediastinal Disorders | |||
| Pharyngitis | 3% | 3% | 3% |
There were no relevant differences in adverse reactions for subgroups of patients as defined by gender, age, or race.
6.2 Post-Marketing Experience
In addition to the adverse reactions reported during clinical trials and listed above, adverse events have been identified during post approval use of CLARINEX-D 12 HOUR Extended Release Tablets. Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Adverse events identified from post-marketing surveillance on the use of CLARINEX-D 12 HOUR Extended Release Tablets include:
Cardiac disorders: tachycardia, palpitations
Respiratory, thoracic and mediastinal disorders: dyspnea
Skin and subcutaneous tissue disorders: rash, pruritus
In addition to these events, the following spontaneous adverse events have been reported during the marketing of desloratadine as a single ingredient product:
Nervous system disorders: headache, somnolence, dizziness, psychomotor hyperactivity, movement disorders (including dystonia, tics, and extrapyramidal symptoms), seizures (reported in patients with and without a known seizure disorder)
Immune system disorders: hypersensitivity reactions (such as urticaria, edema and anaphylaxis)
Investigations: elevated liver enzymes including bilirubin
Hepatobiliary disorders: hepatitis
Metabolism and nutrition disorders: increased appetite
Cases of severe skin reactions such as acute generalized exanthematous pustulosis (AGEP) have been reported with pseudoephedrine-containing products.
Related/similar drugs
7. Drug Interactions
No specific interaction studies have been conducted with CLARINEX-D 12 HOUR Extended Release Tablets.
7.1 Monoamine Oxidase Inhibitors
CLARINEX-D 12 HOUR Extended Release Tablets should not be used in patients receiving monoamine oxidase (MAO) inhibitor therapy or within fourteen (14) days of stopping such treatment because the action of pseudoephedrine a component of CLARINEX-D 12 HOUR Extended Release tablets on the vascular system may be potentiated by these agents [see Contraindications (4) and Warnings and Precautions (5.3)].
7.2 Beta-Adrenergic Blocking Agents
The antihypertensive effects of beta-adrenergic blocking agents, methyldopa, and reserpine, may be reduced by sympathomimetics such as pseudoephedrine. Exercise caution when using CLARINEX-D 12 HOUR Extended Release Tablets with these agents.
7.3 Digitalis
Increased ectopic pacemaker activity can occur when pseudoephedrine is used concomitantly with digitalis. Exercise caution when using CLARINEX-D 12 HOUR Extended Release Tablets with these agents.
7.4 Inhibitors of Cytochrome P450 3A4
In controlled clinical studies co-administration of desloratadine with ketoconazole, erythromycin, or azithromycin resulted in increased plasma concentrations of desloratadine and 3-hydroxydesloratadine but there were no clinically relevant changes in the safety profile of desloratadine [see Clinical Pharmacology (12.3)].
7.5 Fluoxetine
In controlled clinical studies co-administration of desloratadine with fluoxetine, a selective serotonin reuptake inhibitor (SSRI), resulted in increased plasma concentrations of desloratadine and 3-hydroxydesloratadine but there were no clinically relevant changes in the safety profile of desloratadine [see Clinical Pharmacology (12.3)].
7.6 Cimetidine
In controlled clinical studies co-administration of desloratadine with cimetidine a histamine H2-receptor antagonist resulted in increased plasma concentrations of desloratadine and 3-hydroxydesloratadine but there were no clinically relevant changes in the safety profile of desloratadine [see Clinical Pharmacology (12.3)].
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
The limited available data with CLARINEX-D 12 HOUR in pregnant women are not sufficient to inform a drug-associated risk for major birth defects and miscarriage. There are no adequate and well-controlled studies of desloratadine and pseudoephedrine in combination in pregnant women. Neither are there animal reproduction studies conducted with the combination of desloratadine and pseudoephedrine or pseudoephedrine alone. Desloratadine given during organogenesis to pregnant rats was not teratogenic at the summed area under the concentration-time curve (AUC)-based exposures of desloratadine and its metabolite approximately 320 times that at the recommended human daily oral dose (RHD) of 5 mg/day. Desloratadine given during organogenesis to pregnant rabbits was not teratogenic at the AUC-based exposures of desloratadine approximately 230 times that at the RHD. Desloratadine given to pregnant rats during organogenesis through lactation resulted in reduced body weight and slow righting reflex of F1 pups at the summed AUC-based exposures of desloratadine and its metabolite approximately 70 times or greater than that at the RHD [see Data].
The estimated background risk of major birth defects and miscarriage for the indicated populations is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Human Data
Pseudoephedrine
The majority of studies examining the use of pseudoephedrine in pregnancy did not find an association with an increased risk of congenital anomalies. A few case-control studies conducted reported potential associations with isolated congenital disorders. However, several similar studies did not find statistically significant associations. Methodological limitations of these studies included small sample size, selection bias, recall bias, inadequate adjustment for risk factors, residual confounding, exposure misclassification, and lack of information regarding dose and timing of exposure.
Animal Data
No animal reproduction studies were conducted with the combination of desloratadine and pseudoephedrine or pseudoephedrine alone.
Desloratadine
Desloratadine was given orally during organogenesis to pregnant rats at doses of 6, 24 and 48 mg/kg/day (approximately 50, 200 and 320 times the summed AUC-based exposure of desloratadine and its metabolite at the RHD). No fetal malformations were present. Reduced fetal weights and skeletal variations noted at doses of 24 and 48 mg/kg/day were likely secondary to the maternal toxicities of reduced body weight gain and food consumption observed at the same doses. Desloratadine was also given orally during organogenesis to pregnant rabbits at doses of 15, 30 and 60 mg/kg/day (approximately 30, 70 and 230 times the AUC-based exposure of desloratadine at the RHD). No adverse effects to the fetus were noted. Reduced maternal body weight gain was noted in rabbits at 60 mg/kg/day. In a peri- and post-natal development study, desloratadine was given to rats orally during the peri-natal (Gestation Day 6) through lactation periods (Postpartum Day 21) at doses of 3, 9 and 18 mg/kg/day. Reduced body weight and slow righting reflex were reported in F1 pups at doses of 9 mg/kg/day or greater (approximately 70 times or greater than the summed AUC-based exposure of desloratadine and its metabolite at the RHD). Desloratadine had no effect on F1 pup development at 3 mg/kg/day (approximately 10 times the summed AUC-based exposure of desloratadine and its metabolite at the RHD). Maternal toxicities including reduced body weight gain and food consumption were noted at 18 mg/kg/day for F0 dams. F1 offspring were subsequently mated and there was no developmental toxicity for F2 pups observed.
8.2 Lactation
Risk Summary
Desloratadine and pseudoephedrine both pass into breast milk. There are not sufficient data on the effects of desloratadine on the breastfed infant or the effects of desloratadine on milk production. Pseudoephedrine has been reported to decrease milk production [see Data]. Pseudoephedrine has been reported to cause irritability in breastfed infants. The decision should be made whether to discontinue nursing or to discontinue CLARINEX-D 12 HOUR Extended Release Tablets, taking into account the developmental and health benefits of breastfeeding, the nursing mother's clinical need, and any potential adverse effects on the breastfed infant from desloratadine and pseudoephedrine or from the underlying maternal condition.
Data
Human Data
Pseudoephedrine
In a study of eight lactating women, who were 8 to 76 weeks postpartum and received a single dose of 60 mg of pseudoephedrine, the mean 24-hour milk production was reduced by 24%. In the same study, the estimated mean relative infant dose from breast milk (assuming mean milk consumption of 150 mL/kg/day and a maternal dosing regimen of 60 mg pseudoephedrine four times per day) was calculated to be 4.3% of the weight-adjusted maternal dose.
8.3 Females and Males of Reproductive Potential
Infertility
There are no data available on human infertility associated with desloratadine, pseudoephedrine, or the combination. There are no animal fertility studies with the combination or pseudoephedrine alone.
There were no clinically relevant effects of desloratadine on female fertility in rats. A male specific decrease in fertility occurred at an oral desloratadine dose of 12 mg/kg or greater in rats (approximately 65 times the summed AUC-based exposure of desloratadine and its metabolite at the RHD). Male fertility was unaffected at a desloratadine dose of 3 mg/kg (approximately 10 times the summed AUC-based exposure of desloratadine and its metabolite at the RHD). [See Nonclinical Toxicology (13.1).]
8.4 Pediatric Use
CLARINEX-D 12 HOUR Extended Release Tablets are not indicated for use in pediatric patients under 12 years of age.
8.5 Geriatric Use
The number of subjects (n=10) ≥65 years old treated with CLARINEX-D 12 HOUR Extended Release Tablets was too limited to make any formal statistical comparison regarding the efficacy or safety of this drug product in this age group, or to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences between the elderly and younger patients, although the elderly are more likely to have adverse reactions to sympathomimetic amines. In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy [see Clinical Pharmacology (12.3)].
Pseudoephedrine, desloratadine, and their metabolites are known to be substantially excreted by the kidney, and the risk of adverse reactions may be greater in patients with renal impairment. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor the patient for adverse events [see Clinical Pharmacology (12.3)].
8.6 Renal Impairment
No studies with CLARINEX-D 12 HOUR Extended Release Tablets were conducted in subjects with renal impairment.
CLARINEX-D 12 HOUR Extended Release Tablets should generally be avoided in patients with renal impairment [see Warnings and Precautions (5.5) and Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
No studies with CLARINEX-D 12 HOUR Extended Release Tablets or pseudoephedrine were conducted in subjects with hepatic impairment.
CLARINEX-D 12 HOUR Extended Release Tablets should generally be avoided in patients with hepatic impairment [see Warnings and Precautions (5.6) and Clinical Pharmacology (12.3)].
9. Drug Abuse and Dependence
There is no information to indicate that abuse or dependency occurs with CLARINEX or CLARINEX-D 12 HOUR Extended Release Tablets.
10. Overdosage
In the event of overdose, consider standard measures to remove any unabsorbed drug. Symptomatic and supportive treatment is recommended. Desloratadine and 3-hydroxydesloratadine are not eliminated by hemodialysis.
10.1 Desloratadine
Information regarding acute overdosage with desloratadine is limited to experience from post-marketing adverse event reports and from clinical trials conducted during the development of the CLARINEX product. In the reported cases of overdose, there were no significant adverse events that were attributed to desloratadine. In a dose-ranging trial, at doses of 10 mg and 20 mg/day, somnolence was reported.
In another study, no clinically relevant adverse events were reported in normal male and female volunteers who were given single daily doses of CLARINEX 45 mg for 10 days [see Clinical Pharmacology (12.2)].
10.2 Sympathomimetics
In large doses, sympathomimetics such as pseudoephedrine may give rise to giddiness, headache, nausea, vomiting, sweating, thirst, tachycardia, precordial pain, palpitations, difficulty in micturition, muscle weakness and tenseness, anxiety, restlessness, and insomnia. Many patients can present a toxic psychosis with delusions and hallucinations. Some may develop cardiac arrhythmias, circulatory collapse, convulsions, coma, and respiratory failure.
11. Clarinex-D 12 Hour Description
CLARINEX-D 12 HOUR Extended Release Tablets are oval-shaped blue and white bilayer tablets containing 2.5 mg desloratadine in the blue immediate-release layer and 120 mg of pseudoephedrine sulfate USP in the white extended-release layer which is released slowly, allowing for twice-daily administration.
The inactive ingredients contained in CLARINEX-D 12 HOUR Extended Release Tablets are hypromellose USP, microcrystalline cellulose NF, povidone USP, silicon dioxide NF, magnesium stearate NF, corn starch NF, edetate disodium USP, citric acid anhydrous USP, stearic acid NF, and FD&C Blue No. 2 aluminum lake dye.
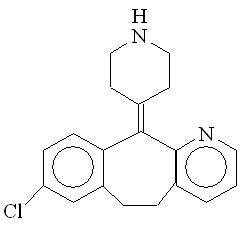
Desloratadine, 1 of the 2 active ingredients of CLARINEX-D 12 HOUR Extended Release Tablets, is a white to off-white powder that is slightly soluble in water, but very soluble in ethanol and propylene glycol. It has an empirical formula: C19H19ClN2 and a molecular weight of 310.8. The chemical name is 8-chloro-6,11-dihydro-11-(4-piperdinylidene)-5H-benzo[5,6] cyclohepta [1,2-b]pyridine and has the following structure:
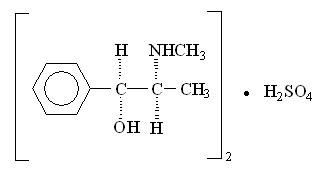
Pseudoephedrine sulfate, the other active ingredient of CLARINEX-D 12 HOUR Extended Release Tablets, is the synthetic salt of one of the naturally occurring dextrorotatory diastereomers of ephedrine and is classified as an indirect sympathomimetic amine. Pseudoephedrine sulfate is a colorless hygroscopic crystal or white, hygroscopic crystalline powder, practically odorless, with a bitter taste. It is very soluble in water, freely soluble in alcohol, and sparingly soluble in ether. The empirical formula for pseudoephedrine sulfate is (C10H15NO)2 • H2SO4; the chemical name is benzenemethanol, α-[1-(methylamino) ethyl]-,[S-(R*,R*)]-, sulfate (2:1)(salt); and the chemical structure is:
12. Clarinex-D 12 Hour - Clinical Pharmacology
12.1 Mechanism of Action
Desloratadine is a long acting tricyclic histamine antagonist with selective H1-receptor histamine antagonist activity. Receptor binding data indicate that at a concentration of 2 to 3 ng/mL (7 nanomolar), desloratadine shows significant interaction with the human histamine H1 receptor. Desloratadine inhibited histamine release from human mast cells in vitro. Results of a radiolabeled tissue distribution study in rats and a radioligand H1-receptor-binding study in guinea pigs showed that desloratadine does not readily cross the blood brain barrier. The clinical significance of this finding is unknown.
Pseudoephedrine sulfate is an orally active sympathomimetic amine and exerts a decongestant action on the nasal mucosa. Pseudoephedrine sulfate is recognized as an effective agent for the relief of nasal congestion due to allergic rhinitis. Pseudoephedrine produces peripheral effects similar to those of ephedrine and central effects similar to, but less intense than, amphetamines. It has the potential for excitatory side effects.
12.2 Pharmacodynamics
Wheal and Flare: Human histamine skin wheal studies following single and repeated 5 mg doses of desloratadine have shown that the drug exhibits an antihistaminic effect by 1 hour; this activity may persist for as long as 24 hours. There was no evidence of histamine-induced skin wheal tachyphylaxis within the desloratadine 5 mg group over the 28-day treatment period. The clinical relevance of histamine wheal skin testing is unknown.
Effects on QTc: In clinical trials for CLARINEX-D 12 HOUR Extended Release Tablets, ECGs were recorded at baseline and endpoint within 1 to 3 hours after the last dose. The majority of ECGs were normal at both baseline and endpoint. No clinically meaningful changes were observed following treatment with CLARINEX-D 12 HOUR Extended Release Tablets for any ECG parameter, including the QTc interval. An increase in the ventricular rate of 7.1 and 6.4 bpm was observed in the CLARINEX-D 12 HOUR Extended Release Tablets and pseudoephedrine groups, respectively, compared to an increase of 3.2 bpm in subjects receiving desloratadine alone. Single daily doses of CLARINEX 45 mg were given to normal male and female volunteers for 10 days.
All ECGs obtained in this study were manually read in a blinded fashion by a cardiologist. In the CLARINEX-treated subjects, there was a mean increase in the maximum heart rate of 9.2 bpm relative to placebo. The QT interval was corrected for heart rate (QTc) by both Bazett's and Fridericia methods. Using the QTc (Bazett), there was a mean increase of 8.1 msec in the CLARINEX-treated subjects relative to placebo. Using QTc (Fridericia) there was a mean increase of 0.4 msec in CLARINEX-treated subjects relative to placebo. No clinically relevant adverse events were reported.
12.3 Pharmacokinetics
Absorption: In a single dose pharmacokinetic study, the mean time to maximum plasma concentrations (Tmax) for desloratadine occurred at approximately 4 to 5 hours post dose and mean peak plasma concentrations (Cmax) and area under the concentration-time curve (AUC) of approximately 1.09 ng/mL and 31.6 ng∙hr/mL, respectively, were observed. In another pharmacokinetic study, food and grapefruit juice had no effect on the bioavailability (Cmax and AUC) of desloratadine.
For pseudoephedrine, the mean Tmax occurred at 6 to 7 hours post dose and mean peak plasma concentrations (Cmax) and area under the concentration-time curve (AUC) of approximately 263 ng/mL and 4588 ng∙hr/mL, respectively, were observed. Food had no effect on the bioavailability (Cmax and AUC) of pseudoephedrine.
Following oral administration of CLARINEX-D 12 HOUR Extended Release Tablets twice daily for 14 days in healthy volunteers, steady-state conditions were reached on Day 10 for desloratadine, 3-hydroxydesloratadine and pseudoephedrine. For desloratadine, mean steady-state peak plasma concentrations (Cmax) and area under the concentration-time curve AUC 0-12 hrs of approximately 1.7 ng/mL and 16 ng∙hr/mL were observed, respectively. For pseudoephedrine, mean steady-state peak plasma concentrations (Cmax) and AUC 0-12 hrs of 459 ng/mL and 4658 ng∙hr/mL were observed.
Distribution: Desloratadine and 3-hydroxydesloratadine are approximately 82% to 87% and 85% to 89%, bound to plasma proteins, respectively. Protein binding of desloratadine and 3-hydroxydesloratadine was unaltered in subjects with impaired renal function.
Metabolism: Desloratadine (a major metabolite of loratadine) is extensively metabolized to 3-hydroxydesloratadine, an active metabolite, which is subsequently glucuronidated. The enzyme(s) responsible for the formation of 3-hydroxydesloratadine have not been identified. Data from clinical trials with desloratadine indicate that a subset of the general population has a decreased ability to form 3-hydroxydesloratadine, and are poor metabolizers of desloratadine. In pharmacokinetic studies (n=3748), approximately 6% of subjects were poor metabolizers of desloratadine (defined as a subject with an AUC ratio of 3-hydroxydesloratadine to desloratadine less than 0.1, or a subject with a desloratadine half-life exceeding 50 hours). These pharmacokinetic studies included subjects between the ages of 2 and 70 years, including 977 subjects aged 2 to 5 years, 1575 subjects aged 6 to 11 years, and 1196 subjects aged 12 to 70 years. There was no difference in the prevalence of poor metabolizers across age groups. The frequency of poor metabolizers was higher in Blacks (17%, n=988) as compared to Caucasians (2%, n=1462) and Hispanics (2%, n=1063). The median exposure (AUC) to desloratadine in the poor metabolizers was approximately 6-fold greater than in the subjects who are not poor metabolizers. Subjects who are poor metabolizers of desloratadine cannot be prospectively identified and will be exposed to higher levels of desloratadine following dosing with the recommended dose of desloratadine. In multidose clinical safety studies, where metabolizer status was prospectively identified, a total of 94 poor metabolizers and 123 normal metabolizers were enrolled and treated with CLARINEX Syrup for 15 to 35 days. In these studies, no overall differences in safety were observed between poor metabolizers and normal metabolizers. Although not seen in these studies, an increased risk of exposure-related adverse events in patients who are poor metabolizers cannot be ruled out.
Pseudoephedrine alone is incompletely metabolized (less than 1%) in the liver by N-demethylation to an inactive metabolite. The drug and its metabolite are excreted in the urine. About 55% to 96% of an administered dose of pseudoephedrine hydrochloride is excreted unchanged in the urine.
Elimination: Following single dose administration of CLARINEX-D 12 HOUR Extended Release Tablets, the mean plasma elimination half-life of desloratadine was approximately 27 hours. In another study, following administration of single oral doses of desloratadine 5 mg, Cmax and AUC values increased in a dose proportional manner following single oral doses between 5 and 20 mg. The degree of accumulation after 14 days of dosing was consistent with the half-life and dosing frequency. A human mass balance study documented a recovery of approximately 87% of the 14C-desloratadine dose, which was equally distributed in urine and feces as metabolic products. Analysis of plasma 3-hydroxydesloratadine showed similar Tmax and half-life values compared to desloratadine.
The mean elimination half-life of pseudoephedrine is dependent on urinary pH. The elimination half-life is approximately 3 to 6 or 9 to 16 hours when the urinary pH is 5 or 8, respectively.
Geriatric Subjects: Following multiple-dose administration of CLARINEX Tablets, the mean Cmax and AUC values for desloratadine were 20% greater than in younger subjects (< 65 years old). The oral total body clearance (CL/F) when normalized for body weight was similar between the 2 age groups. The mean plasma elimination half-life of desloratadine was 33.7 hr in subjects ≥65 years old. The pharmacokinetics for 3-hydroxydesloratadine appeared unchanged in older vs. younger subjects. These age-related differences are unlikely to be clinically relevant and no dosage adjustment is recommended in elderly patients.
Pediatric Subjects: CLARINEX-D 12 HOUR Extended Release Tablets are not an appropriate dosage form for use in pediatric patients below 12 years of age.
Renally Impaired: Following a single dose of desloratadine 7.5 mg, pharmacokinetics were characterized in subjects with mild (n=7; creatinine clearance 51–69 mL/min/1.73 m2), moderate (n=6; creatinine clearance 34–43 mL/min/1.73 m2) and severe (n=6; creatinine clearance 5–29 mL/min/1.73 m2) renal impairment or hemodialysis dependent (n=6) subjects. In subjects with mild and moderate renal impairment, median Cmax and AUC values increased by approximately 1.2- and 1.9-fold, respectively, relative to subjects with normal renal function. In subjects with severe renal impairment or who were hemodialysis dependent, Cmax and AUC values increased by approximately 1.7- and 2.5-fold, respectively. Minimal changes in 3-hydroxydesloratadine concentrations were observed. Desloratadine and 3-hydroxydesloratadine were poorly removed by hemodialysis. Plasma protein binding of desloratadine and 3-hydroxydesloratadine was unaltered by renal impairment.
Pseudoephedrine is primarily excreted unchanged in the urine as unchanged drug with the remainder apparently being metabolized in the liver. Therefore, pseudoephedrine may accumulate in patients with renal impairment.
Hepatically Impaired: Following a single oral dose of desloratadine, pharmacokinetics were characterized in subjects with mild (n=4), moderate (n=4) and severe (n=4) hepatic impairment as defined by the Child-Pugh classification of hepatic impairment and 8 subjects with normal hepatic function. Subjects with hepatic impairment, regardless of severity, had approximately a 2.4-fold increase in AUC as compared with normal subjects. The apparent oral clearance of desloratadine in subjects with mild, moderate, and severe hepatic impairment was 37%, 36%, and 28% of that in normal subjects, respectively. An increase in the mean elimination half-life of desloratadine in subjects with hepatic impairment was observed. For 3-hydroxydesloratadine, the mean Cmax and AUC values for subjects with hepatic impairment combined were not statistically significantly different from subjects with normal hepatic function.
Gender: Female subjects treated for 14 days with CLARINEX Tablets had 10% and 3% higher desloratadine Cmax and AUC values, respectively, compared with male subjects. The 3-hydroxydesloratadine Cmax and AUC values were also increased by 45% and 48%, respectively, in females compared with males. However, these apparent differences are not considered to be clinically relevant.
Race: Following 14 days of treatment with CLARINEX Tablets, the Cmax and AUC values for desloratadine were 18% and 32% higher, respectively in Blacks compared with Caucasians. For 3-hydroxydesloratadine there was a corresponding 10% reduction in Cmax and AUC values in Blacks compared to Caucasians. These differences are not considered to be clinically relevant.
Drug Interaction: In 2 controlled crossover clinical pharmacology studies in healthy male (n=12 in each study) and female (n=12 in each study) subjects, desloratadine 7.5 mg (1.5 times the daily dose) once daily was co-administered with erythromycin 500 mg every 8 hours or ketoconazole 200 mg every 12 hours for 10 days. In 3 separate controlled, parallel group clinical pharmacology studies, desloratadine at the clinical dose of 5 mg has been co-administered with azithromycin 500 mg followed by 250 mg once daily for 4 days (n=18) or with fluoxetine 20 mg once daily for 7 days after a 23-day pretreatment period with fluoxetine (n=18) or with cimetidine 600 mg every 12 hours for 14 days (n=18) under steady state conditions to healthy male and female subjects. Although increased plasma concentrations (Cmax and AUC 0-24 hrs) of desloratadine and 3-hydroxydesloratadine were observed (see Table 2), there were no clinically relevant changes in the safety profile of desloratadine, as assessed by electrocardiographic parameters (including the corrected QT interval), clinical laboratory tests, vital signs and adverse events.
| Desloratadine | 3-hydroxydesloratadine | |||
|---|---|---|---|---|
| Cmax | AUC 0-24hrs | Cmax | AUC 0-24hrs | |
| Erythromycin (500 mg Q8h) | +24% | +14% | +43% | +40% |
| Ketoconazole (200 mg Q12h) | +45% | +39% | +43% | +72% |
| Azithromycin (500 mg Day 1, 250 mg QD × 4 days) | +15% | +5% | +15% | +4% |
| Fluoxetine (20 mg QD) | +15% | +0% | +17% | +13% |
| Cimetidine (600 mg Q12h) | +12% | +19% | -11% | -3% |
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
There are no animal or laboratory studies on the combination product of desloratadine and pseudoephedrine sulfate or pseudoephedrine alone to evaluate carcinogenesis, mutagenesis, or impairment of fertility.
Carcinogenicity Studies:
Desloratadine
The carcinogenic potential of desloratadine was assessed using a loratadine study in rats and a desloratadine study in mice. In a 2-year study in rats, loratadine was administered in the diet at doses up to 25 mg/kg/day (approximately 45 times the summed AUC-based exposure of desloratadine and its metabolite at the RHD). A significantly higher incidence of hepatocellular tumors (combined adenomas and carcinomas) was observed in males given 10 mg/kg/day of loratadine (approximately 10 times the summed AUC-based exposure of desloratadine and its metabolite at the RHD) and in males and females given 25 mg/kg/day of loratadine. The clinical significance of these findings during long-term use of desloratadine is not known. In a 2-year dietary study in mice, males and females given up to 16 mg/kg/day and 32 mg/kg/day desloratadine, respectively (approximately 30 and 70 times the summed AUC-based exposure of desloratadine and its metabolite at the RHD, respectively), did not show significant increases in the incidence of any tumors.
Pseudoephedrine
The carcinogenic potential of pseudoephedrine was assessed using ephedrine sulfate studies in F344/N rats and B6C3F1 mice conducted under the National Toxicology Program (NTP). In a 2-year dietary study in rats, male and female rats given up to 9 and 11 mg/kg/day ephedrine sulfate (approximately 0.4 and 0.5 times the RHD of 240 mg/day on a mg/m2 basis, respectively) did not show any evidence of tumorigenicity. In a 2-year dietary study in mice, male and female mice given up to 29 and 25 mg/kg/day ephedrine sulfate (approximately 0.7 and 0.6 times the RHD of pseudoephedrine on a mg/m2 basis, respectively) did not show any evidence of tumorigenicity.
Genotoxicity Studies:
Desloratadine
In genotoxicity studies with desloratadine, there was no evidence of genotoxic potential in a reverse mutation assay (Salmonella/E. coli mammalian microsome bacterial mutagenicity assay) or in 2 assays for chromosomal aberrations (human peripheral blood lymphocyte clastogenicity assay and mouse bone marrow micronucleus assay).
Impairment of Fertility:
Desloratadine
In a female fertility study, desloratadine was given to female rats orally 14 days prior to and throughout mating until Gestation Day 7 at doses of 6, 12 and 24 mg/kg/day. An increase in preimplantation loss and a decrease in number of implantations and fetuses noted at 24 mg/kg (approximately 200 times the summed AUC-based exposure of desloratadine and its metabolite at the RHD) was likely due to maternal toxicities including reduced body weight gain and food consumption. In a male fertility study in rats, desloratadine was given orally to male rats for 70 days prior to mating and throughout the mating period (total dosing period 106-108 days) at doses of 3, 12 and 40 mg/kg/day. Reduced body weight gain, food consumption, and absolute organ weights of testes, epididymis, and cauda epididymis were noted at 40 mg/kg/day. A male-specific decrease in fertility, demonstrated by reduced female conception rates, decreased sperm numbers and motility, and histopathologic changes in testes and epididymis, occurred at a dose of 12 mg/kg or greater (approximately 65 times or greater than the summed AUC-based exposure of desloratadine and its metabolite at the RHD). Desloratadine had no effect on male fertility in rats at 3 mg/kg/day (approximately 10 times the summed AUC-based exposure of desloratadine and its metabolite at the RHD).
14. Clinical Studies
14.1 Seasonal Allergic Rhinitis
The clinical efficacy and safety of CLARINEX-D 12 HOUR Extended Release Tablets was evaluated in two 2-week multicenter, randomized parallel group clinical trials involving 1248 subjects 12 to 78 years of age with seasonal allergic rhinitis, 414 of whom received CLARINEX-D 12 HOUR Extended Release Tablets. In the 2 trials, subjects were randomized to receive CLARINEX-D 12 HOUR Extended Release Tablets twice daily, CLARINEX Tablets 5 mg once daily, or sustained-release pseudoephedrine tablet 120 mg twice daily for 2 weeks. The majority of patients were between 18 and <65 years of age with a mean age of 35.8 years and were predominantly women (64%). Patient ethnicity was 82% Caucasian, 9% Black, 6% Hispanic and 3% Asian/other ethnicity. Primary efficacy variable was twice-daily reflective patient scoring of 4 nasal symptoms (rhinorrhea, nasal stuffiness/congestion, nasal itching, and sneezing) and four non-nasal symptoms (itching/burning eyes, tearing/watering eyes, redness of eyes, and itching of ears/palate) on a 4 point scale (0=none, 1=mild, 2=moderate, and 3=severe). In both trials, the antihistaminic efficacy of CLARINEX-D 12 HOUR Extended Release Tablets, as measured by total symptom score excluding nasal congestion, was significantly greater than pseudoephedrine alone over the 2-week treatment period; and the decongestant efficacy of CLARINEX-D 12 HOUR Extended Release Tablets, as measured by nasal stuffiness/congestion, was significantly greater than CLARINEX (desloratadine alone) over the 2-week treatment period. Primary efficacy variable results from 1 of 2 trials are shown in Table 3.
| Treatment Group (n) | Mean Baseline*
(SEM) | Change (% Change) from Baseline†
(SEM) | CLARINEX-D 12 HOUR Comparison to Components‡ (P-value) |
|---|---|---|---|
| SEM=Standard Error of the Mean | |||
|
|||
| Total Symptom Score (Excluding Nasal Congestion) | |||
| CLARINEX-D 12 HOUR Extended Release Tablets BID
(199) | 14.18 (0.21) | -6.54 (-46.0) (0.30) | - |
| Pseudoephedrine tablet 120 mg BID
(197) | 14.06 (0.21) | -5.07 (-35.9) (0.30) | P<0.001 |
| CLARINEX 5 mg Tablets QD
(197) | 14.82 (0.21) | -5.09 (-33.5) (0.30) | P<0.001 |
| Nasal Stuffiness/Congestion | |||
| CLARINEX-D 12 HOUR Extended Release Tablets BID
(199) | 2.47 (0.027) | -0.93 (-37.4) (0.046) | - |
| Pseudoephedrine tablet 120 mg BID
(197) | 2.46 (0.027) | -0.75 (-31.2) (0.046) | P=0.006 |
| CLARINEX 5 mg Tablets QD
(197) | 2.50 (0.027) | -0.66 (-26.7) (0.046) | P<0.001
|
There were no significant differences in the efficacy of CLARINEX-D 12 HOUR Extended Release Tablets across subgroups of subjects defined by gender, age, or race.
16. How is Clarinex-D 12 Hour supplied
CLARINEX-D 12 HOUR Extended Release Tablets are oval-shaped, blue and white bilayer tablets with "D12" embossed in the blue layer, containing 2.5 mg desloratadine in the blue immediate-release layer and 120 mg of pseudoephedrine sulfate USP in the white extended-release layer. CLARINEX-D 12 HOUR Extended Release Tablets are supplied in high-density polyethylene bottles of 100 (NDC 78206-120-01).
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information).
17.1 Cardiovascular and Central Nervous System Effects
Patients should be informed that pseudoephedrine, one of the active ingredients in CLARINEX-D 12 HOUR Extended Release Tablets may cause cardiovascular or central nervous system effects such as insomnia, dizziness, tremor, convulsions or arrhythmia.
17.2 Dosing
Patients should be advised not to increase the dose or dosing frequency of CLARINEX-D 12 HOUR Extended Release Tablets.
17.3 Additional Antihistamines and/or Decongestants
Patients should be advised against the concurrent use of CLARINEX-D 12 HOUR Extended Release Tablets with other antihistamines and/or decongestants.
17.4 Monoamine Oxidase (MAO) Inhibitors
Patients should be informed that due to its pseudoephedrine component, they should not use CLARINEX-D 12 HOUR with a monoamine oxidase (MAO) inhibitor or within 14 days of stopping use of an MAO inhibitor.
Manufactured for: Organon LLC, a subsidiary of
ORGANON & Co.,
Jersey City, NJ 07302, USA
For patent information: www.organon.com/our-solutions/patent/
uspi-og4117a-t-d12-2106r000
PATIENT INFORMATION
CLARINEX-D® (CLA-RI-NEX) 12 Hour Extended Release Tablets
(desloratadine and pseudoephedrine sulfate)
Read the Patient Information that comes with CLARINEX-D 12 Hour Extended Release Tablets before you start taking it and each time you get a refill. There may be new information. This leaflet is a summary of the information for patients. Your doctor or pharmacist can give you additional information. This leaflet does not take the place of talking to your doctor about your medical condition or treatment.
What is CLARINEX-D® 12 Hour Extended Release Tablets?
CLARINEX-D 12 Hour Extended Release Tablets is a prescription medicine that contains the medicines desloratadine (an antihistamine) and pseudoephedrine (a nasal decongestant). CLARINEX-D 12 Hour Extended Release Tablets is used to help control the symptoms of seasonal allergic rhinitis (sneezing, stuffy nose, runny nose and itching of the nose) in adults and children 12 years and older.
CLARINEX-D 12 Hour Extended Release Tablets is not for children under 12 years of age.
Who should not take CLARINEX-D® 12 Hour Extended Release Tablets?
Do not take CLARINEX-D 12 Hour Extended Release Tablets if you:
- are allergic to desloratadine or pseudoephedrine sulfate or any of the ingredients in CLARINEX-D 12 Hour Extended Release Tablets. See the end of this leaflet for a complete list of ingredients in CLARINEX-D 12 Hour Extended Release Tablets.
- are allergic to loratadine (Alavert, Claritin)
- have narrow-angle glaucoma
- have problems with urination (urinary retention)
- take a Monoamine Oxidase Inhibitor (MAOI) medicine to treat depression, or if you stopped taking an MAOI medicine within the last 2 weeks. Ask your doctor or pharmacist if you are not sure if you take an MAOI medicine.
- have severe high blood pressure
- have severe heart disease
Talk to your doctor before taking this medicine if you have any of these conditions.
What should I tell my doctor before taking CLARINEX-D® 12 Hour Extended Release Tablets?
Before you take CLARINEX-D 12 Hour Extended Release Tablets, tell your doctor if you:
- have any of the conditions listed in the section "Who should not take CLARINEX-D 12 Hour Extended Release Tablets?"
- diabetes
- hyperthyroidism
- have prostate problems
- have liver or kidney problems
- have any other medical conditions
- are pregnant or plan to become pregnant. It is not known if CLARINEX-D 12 Hour Extended Release Tablets will harm your unborn baby. Talk to your doctor if you are pregnant or plan to become pregnant.
- are breastfeeding or plan to breastfeed. CLARINEX-D 12 Hour Extended Release Tablets can pass into your breast milk. Talk to your doctor about the best way to feed your baby if you take CLARINEX-D 12 Hour Extended Release Tablets.
Tell your doctor about all the medicines you take, including prescription and nonprescription medicines, vitamins and herbal supplements. CLARINEX-D® 12 Hour Extended Release Tablets may affect the way other medicines work, and other medicines may affect how CLARINEX-D 12 Hour Extended Release Tablets works. Especially tell your doctor if you take:
- Monoamine Oxidase Inhibitors (MAOIs). You should not use CLARINEX-D 12 Hour Extended Release Tablets if you take an MAOI or within 2 weeks of stopping an MAOI.
- methyldopa
- reserpine (Serpalan)
- digitalis (Digoxin, Lanoxicaps, Lanoxin) ketoconazole (Nizoral)
- erythromycin (Ery-tab, Eryc, PCE)
- azithromycin (Zithromax, Zmax)
- antihistamines
- other decongestant medicines
Know the medicines you take. Keep a list of your medicines and show it to your doctor and pharmacist when you get a new medicine.
How should I take CLARINEX-D® 12 Hour Extended Release Tablets?
Take CLARINEX-D 12 Hour Extended Release Tablets exactly as your doctor tells you to take it.
- CLARINEX-D 12 Hour Extended Release Tablets can be taken with or without food.
- Swallow CLARINEX-D 12 Hour Extended Release Tablets whole. Do not break, crush, or chew CLARINEX-D 12 Hour Extended Release Tablets before swallowing. If you cannot swallow CLARINEX-D 12 Hour Extended Release Tablets whole, tell your doctor. You may need a different medicine.
- Take 1 CLARINEX-D 12 Hour Extended Release Tablet 2 times a day (every 12 hours).
What are the possible side effects of CLARINEX-D® 12 Hour Extended Release Tablets?
CLARINEX-D 12 Hour Extended Release Tablets may cause serious side effects, including:
- Cardiovascular and central nervous system effects, such as
- unable to sleep (insomnia)
- dizziness
- weakness
- tremor
- irregular heart beat
- seizure
- low blood pressure
- Increased sleepiness or tiredness can happen if you take more CLARINEX-D 12 Hour Extended Release Tablets than your doctor prescribed to you.
- Allergic reactions. Stop taking CLARINEX-D 12 Hour Extended Release Tablets and call your doctor right away or get emergency help if you have any of these symptoms:
- rash
- itching
- hives
- swelling of your lips, tongue, face, and throat
- shortness of breath or trouble breathing
- Severe skin reactions including signs and symptoms such as fever, reddening of the skin, or many small pimples
The most common side effects of CLARINEX-D 12 HOUR Extended Release Tablets include:
- unable to sleep (insomnia)
- sore throat
- headache
- dizziness
- dry mouth
- nausea
- tiredness
- loss of appetite
- sleepiness
Tell your doctor if you have any side effect that bothers you or that does not go away.
These are not all of the possible side effects of CLARINEX-D 12 Hour Extended Release Tablets. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store CLARINEX-D® 12 Hour Extended Release Tablets?
- Store CLARINEX-D 12 Hour Extended Release Tablets at 59°F to 86°F (15°C to 30°C)
- Keep CLARINEX-D 12 Hour Extended Release Tablets dry and out of the light.
Keep CLARINEX-D 12 Hour Extended Release Tablets and all medicines out of the reach of children.
General information about CLARINEX-D® 12 Hour Extended Release Tablets
Medicines are sometimes prescribed for purposes other than those listed in a patient information leaflet. Do not use CLARINEX-D 12 Hour Extended Release Tablets for a condition for which it was not prescribed. Do not give CLARINEX-D 12 Hour Extended Release Tablets to other people, even if they have the same condition you have. It may harm them.
This patient information leaflet summarizes the most important information about CLARINEX-D 12 Hour Extended Release Tablets. If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about CLARINEX-D 12 Hour Extended Release Tablets that is written for health professionals.
What are the ingredients in CLARINEX-D® 12 Hour Extended Release Tablets?
Active ingredients: desloratadine and pseudoephedrine sulfate
Inactive ingredients: hypromellose USP, microcrystalline cellulose NF, povidone USP, silicon dioxide NF, magnesium stearate NF, corn starch NF, edetate disodium USP, citric acid anhydrous USP, stearic acid NF, and FD&C Blue No. 2 aluminum lake dye.
| CLARINEX-D 12 HOUR
desloratadine and pseudoephedrine sulfate tablet, extended release |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Organon LLC (117494753) |
More about Clarinex-D 12 Hour (desloratadine / pseudoephedrine)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (1)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Drug class: upper respiratory combinations
- En español
Patient resources
Other formulations
Related treatment guides
Copyright © 2021 Organon Global Inc.
All rights reserved.