Sipuleucel-T
Medically reviewed by Drugs.com. Last updated on Nov 11, 2024.
Pronunciation
(si pu LOO sel tee)
Index Terms
- APC8015
- Prostate Cancer Vaccine, Cell-Based
Dosage Forms
Excipient information presented when available (limited, particularly for generics); consult specific product labeling.
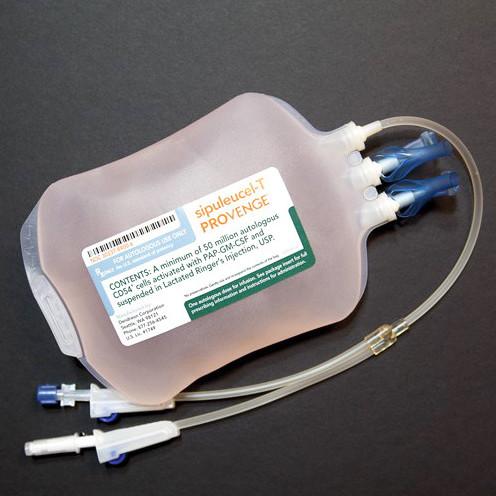
Suspension, Intravenous [preservative free]:
Provenge: (250 mL)
Brand Names: U.S.
- Provenge
Pharmacologic Category
- Cellular Immunotherapy, Autologous
Pharmacology
Sipuleucel-T is an autologous cellular immunotherapy that stimulates an immune response against an antigen (prostatic acid phosphatase [PAP]) expressed in most prostate cancer tissues. Peripheral blood is collected (~3 days prior to infusion) from the patient via leukapheresis, from which peripheral blood mononuclear cells (PBMCs) are isolated. Antigen presenting cell (APC) precursors, consisting of CD54-positive cells that include dendritic cells, are isolated from the PBMCs. The APCs are then activated (in vitro) with a recombinant human fusion protein, PAP-GM-CSF (also termed PA2024), composed of an antigen specific for prostate cancer, PAP linked to granulocyte-macrophage colony-stimulating factor and cultured for ~40 hours. The final product, sipuleucel-T, is reinfused into the patient, inducing T-cell immunity to tumors that express PAP.
Use: Labeled Indications
Prostate cancer, metastatic: Treatment of asymptomatic or minimally symptomatic metastatic castrate-resistant (hormone-refractory) prostate cancer.
Contraindications
There are no contraindications listed in the manufacturer’s labeling.
Dosing: Adult
Note: Premedicate with oral acetaminophen 650 mg and an antihistamine (eg, diphenhydramine 50 mg) ~30 minutes prior to infusion. For autologous use only. Do not infuse until confirmation of product release has been received from the manufacturing company.
Prostate cancer, metastatic: IV: Each dose contains ≥50 million autologous CD54+ cells (obtained through leukapheresis) activated with PAP-GM-CSF; administer doses at ~2-week intervals for a total of 3 doses (Kantoff 2010). If unable to receive a scheduled infusion, an additional leukapheresis procedure will be necessary prior to continuing a course of treatment.
Dosing: Geriatric
Refer to adult dosing.
Dosing: Adjustment for Toxicity
Acute infusion reaction: Interrupt or slow infusion rate (depending on the severity of infusion reaction); may require acetaminophen, IV H1 and/or H2 antagonists, or low-dose meperidine to manage acute symptoms.
Reconstitution
Sipuleucel-T will arrive as a prepared patient-specific 250 mL suspension in lactated Ringer’s injection. Contents may appear clear to opaque and will be a white to red color, including shades of off-white, cream, light yellow, and orange. If clumps or clots are present, gently mix to resuspend. Do not administer if the bag leaks during handling, is damaged, or if clumps remain.
Administration
IV: For autologous use only; the identity of the patient must be matched to the patient identifiers on the infusion bag and on the “Final Product Disposition Notification” prior to infusion. Do not infuse until confirmation of product release is received from the manufacturing company. Keep the sealed infusion bag in the insulated polyurethane container inside the shipping box until ready for administration. Prior to infusion, inspect bag for signs of leaks (do not administer if leaking) or damage. Gently mix to resuspend contents; inspect for clumps or clotting; small clumps should disperse with the gentle mixing; do not administer if clumps remain. Infusion must begin prior to the expiration date and time; do NOT infuse if expired.
For IV infusion only. Infuse over ~60 minutes; infuse the entire contents of the bag. Do NOT use a cell filter for infusion. Premedicate with oral acetaminophen and diphenhydramine ~30 minutes prior to infusion. If acute infusion reaction occurs, interrupt or slow infusion rate (depending on the severity of infusion reaction); may require acetaminophen, IV H1 and/or H2 antagonists, or low-dose meperidine to manage acute symptoms. If infusion is interrupted, keep infusion bag at room temperature; do not resume if bag is retained at room temperature for >3 hours. Observe patient for at least 30 minutes after infusion.
Storage
Store in cardboard shipping carton upon arrival (the insulated container and gel packs are designed to maintain appropriate storage temperature). Do not remove the infusion bag from the insulated polyurethane container within the shipping box until administration (do not remove the insulated container from the shipping box, or open the lid of the insulated container, until administration). Product may only remain at room temperature for ≤3 hours once removed from shipping container; after removal from shipping container, do not return product to container. Infusion must begin prior to product expiration.
Drug Interactions
Immunosuppressants: May diminish the therapeutic effect of Sipuleucel-T. Management: Evaluate patients to see if it is medically appropriate to reduce or discontinue therapy with immunosuppressants prior to initiating sipuleucel-T therapy. Consider therapy modification
Adverse Reactions
The following adverse drug reactions and incidences are derived from product labeling unless otherwise specified.
Note: Initial infusion-related events usually present within the first 24 hours after administration.
>10%:
Central nervous system: Chills (53%; grades ≥3: 2%), fatigue (41%; grades ≥3: 1%), headache (18%; grades ≥3: <1%), dizziness (12%; grades ≥3: <1%), pain (12%)
Gastrointestinal: Nausea (22%; grades ≥3: <1%), vomiting (13% grades ≥3: <1%), constipation (12%; grades ≥3: <1%)
Hematologic: Anemia (13%)
Hypersensitivity: Severe infusion related reaction (71%; grade 3: 4%)
Neuromuscular & skeletal: Back pain (30%; grades ≥3: 3%), myalgia (12%; grades ≥3: <1%), weakness (11%; grades ≥3: 1%)
Miscellaneous: Fever (31%; grades ≥3: 1%), citrate toxicity (15%)
1% to 10%:
Cardiovascular: Hypertension (8% grades ≥3: <1%), hemorrhagic stroke (4%)
Dermatologic: Diaphoresis (5%; grades ≥3: <1%), skin rash (5%)
Gastrointestinal: Anorexia (7%), acute ischemic stroke (4%)
Genitourinary: Hematuria (8%)
Neuromuscular & skeletal: Musculoskeletal pain (9%; grades ≥3: <1%), muscle spasm (8%; grades ≥3: <1%), neck pain (6%), tremor (5%)
Renal: Hematuria (8%)
Respiratory: Flu-like symptoms (10%), dyspnea (9%; grades ≥3: 2%)
<1%, postmarketing, and/or case reports: Cerebrovascular accident, eosinophilia, hypotension, myasthenia gravis, myocardial infarction, myositis, paresthesia (grades ≥3), pulmonary embolism, rhabdomyolysis, sepsis, syncope, transient ischemic attacks, tumor flare, venous thrombosis
Related/similar drugs
Warnings/Precautions
Concerns related to adverse effects:
• Infusion reaction: Acute infusion reactions may occur within 1 day of infusion and are usually mild or moderate for most patients; the incidence of severe reaction may be higher with the second infusion, while the third infusion is associated with a decrease in the incidence of severe reactions. Premedication with oral acetaminophen and diphenhydramine is recommended. Depending on the severity of the infusion reaction, interrupt or slow infusion rate; in clinical trials, acetaminophen, IV H1 and/or H2 antagonists, and low-dose meperidine were used to manage acute symptoms. Symptoms of acute infusion reaction may include chills, rigor, fever, bronchospasm, dyspnea, hypoxia, hypertension, tachycardia, syncope, hypotension, joint or muscle aches, nausea, vomiting, dizziness, fatigue, headache, and weakness; fever and chills usually resolved within 2 days. Observe patient for at least 30 minutes after infusion. Closely monitor patients with cardiac or pulmonary conditions during infusion.
• Thromboembolic events: Deep venous thrombosis and pulmonary embolism occurred following sipuleucel-T infusion (postmarketing reports), usually in patients with multiple risk factors for thromboembolism. Use with caution in patients at risk for thromboembolic events.
• Vascular disorders: Cerebrovascular (hemorrhagic and ischemic stroke) and cardiovascular events (myocardial infarction) have occurred; transient ischemic attacks have been reported following infusion (postmarketing reports). Such events usually occurred in patients with multiple risk factors for cerebrovascular or cardiovascular incidents.
Concurrent drug therapy issues:
• Androgen deprivation therapy: In clinical trials evaluating sipuleucel-T, patients who had androgen deprivation therapy without prior bilateral orchiectomy were continued on gonadal suppression with a luteinizing hormone-releasing hormone agonist (Higano 2009; Kantoff 2010).
• Chemotherapy: Concurrent use with chemotherapy has not been studied.
• Immunosuppressive therapy: Concurrent use with immunosuppressives (eg, corticosteroids) has not been studied; may alter the efficacy and/or safety of sipuleucel-T. Carefully evaluate patients for appropriateness of reducing or discontinuing immunosuppressive agents prior to treatment.
Other warnings/precautions:
• Appropriate use: For autologous use only. Patient identity must be matched to the patient identifiers on the infusion bag and on the Final Product Disposition Notification (provided by manufacturer) prior to infusion. Confirmation of product release must be received from the manufacturer prior to infusion.
• Handling precautions: Apply universal precautions for product handling. Sipuleucel-T is not tested for transmissible infectious diseases; patient-specific leukapheresis collection and activated product may have a risk for infectious disease transmission.
• Sterility testing: Preliminary sterility testing is done based on a 2-day incubation period. Final (7-day incubation) testing is not available until after administration; health care providers will be notified if 7-day sterility tests are positive for microbial contamination.
• Treatment delays: If unable to receive a scheduled reinfusion, an additional leukapheresis procedure may be required; advise patients of this possibility before treatment initiation.
Monitoring Parameters
Monitor for infusion reaction during and for at least 30 minutes after infusion; monitor closely during infusion for patients with cardiovascular and pulmonary disease; monitor for thromboembolic and vascular events.
Pregnancy Considerations
Animal reproduction studies have not been conducted. Not indicated for use in women.
Patient Education
What is this drug used for?
• It is used to treat prostate cancer.
• It may be given to you for other reasons. Talk with the doctor.
All drugs may cause side effects. However, many people have no side effects or only have minor side effects. Call your doctor or get medical help if any of these side effects or any other side effects bother you or do not go away:
• Constipation
• Lack of appetite
• Bone pain
• Common cold symptoms
• Trouble sleeping
• Neck pain
• Weight loss
• Sweating a lot
• Tremor
• Muscle spasm
• Diarrhea
• Flushing
• Joint pain
• Back pain
WARNING/CAUTION: Even though it may be rare, some people may have very bad and sometimes deadly side effects when taking a drug. Tell your doctor or get medical help right away if you have any of the following signs or symptoms that may be related to a very bad side effect:
• Infection
• Urinary tract infection like blood in the urine, burning or painful urination, passing a lot of urine, fever, lower abdominal pain, or pelvic pain
• Weakness on 1 side of the body, trouble speaking or thinking, change in balance, drooping on one side of the face, or blurred eyesight
• Blood clots like numbness or weakness on one side of the body; pain, redness, tenderness, warmth, or swelling in the arms or legs; change in color of an arm or leg; chest pain; shortness of breath; fast heartbeat; or coughing up blood
• Burning or numbness feeling
• Shortness of breath
• Passing out
• Dizziness
• Fast heartbeat
• Chest pain
• Abnormal heartbeat
• Swelling of arms or legs
• Flu-like symptoms
• Severe loss of strength and energy
• Severe nausea
• Vomiting
• Headache
• Muscle pain
• Injection site pain, swelling, or irritation
• Signs of an allergic reaction, like rash; hives; itching; red, swollen, blistered, or peeling skin with or without fever; wheezing; tightness in the chest or throat; trouble breathing, swallowing, or talking; unusual hoarseness; or swelling of the mouth, face, lips, tongue, or throat.
Note: This is not a comprehensive list of all side effects. Talk to your doctor if you have questions.
Consumer Information Use and Disclaimer: This information should not be used to decide whether or not to take this medicine or any other medicine. Only the healthcare provider has the knowledge and training to decide which medicines are right for a specific patient. This information does not endorse any medicine as safe, effective, or approved for treating any patient or health condition. This is only a limited summary of general information about the medicine's uses from the patient education leaflet and is not intended to be comprehensive. This limited summary does NOT include all information available about the possible uses, directions, warnings, precautions, interactions, adverse effects, or risks that may apply to this medicine. This information is not intended to provide medical advice, diagnosis or treatment and does not replace information you receive from the healthcare provider. For a more detailed summary of information about the risks and benefits of using this medicine, please speak with your healthcare provider and review the entire patient education leaflet.
Biological Products Related to sipuleucel-T
Find detailed information on biosimilars for this medication.
More about sipuleucel-T
- Check interactions
- Compare alternatives
- Reviews (7)
- Side effects
- Dosage information
- During pregnancy
- Drug class: therapeutic vaccines
- En español
Patient resources
Other brands
Professional resources
Other brands
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.