Nplate Dosage
Generic name: ROMIPLOSTIM 500ug in 1mL
Dosage form: injection, powder, lyophilized, for solution
Drug class: Platelet-stimulating agents
Medically reviewed by Drugs.com. Last updated on Mar 13, 2025.
Patients with Immune Thrombocytopenia (ITP)
Use the lowest dose of Nplate to achieve and maintain a platelet count ≥ 50 × 109/L as necessary to reduce the risk for bleeding. Administer Nplate as a weekly subcutaneous injection with dose adjustments based upon the platelet count response.
The prescribed Nplate dose may consist of a very small volume (e.g., 0.15 mL). Administer Nplate only with a syringe that contains 0.01 mL graduations.
Discontinue Nplate if the platelet count does not increase to a level sufficient to avoid clinically important bleeding after 4 weeks of Nplate therapy at the maximum weekly dose of 10 mcg/kg.
Obtain complete blood counts (CBCs), including platelet counts, weekly during the dose adjustment phase of Nplate therapy and then monthly following establishment of a stable Nplate dose. Obtain CBCs, including platelet counts, weekly for at least 2 weeks following discontinuation of Nplate.
For Adult Patients with ITP
The initial dose of Nplate is 1 mcg/kg. Actual body weight at initiation of treatment should always be used when calculating the initial dose. In adults, future dose adjustments are based on changes in platelet counts only.
Adjust the weekly dose of Nplate by increments of 1 mcg/kg until the patient achieves a platelet count ≥ 50 × 109/L as necessary to reduce the risk for bleeding; do not exceed a maximum weekly dose of 10 mcg/kg. In clinical studies, most adult patients who responded to Nplate achieved and maintained platelet counts ≥ 50 × 109/L with a median dose of 2-3 mcg/kg.
Adjust the dose as follows for adult patients:
- If the platelet count is < 50 × 109/L, increase the dose by 1 mcg/kg.
- If platelet count is > 200 × 109/L and ≤ 400 × 109/L for 2 consecutive weeks, reduce the dose by 1 mcg/kg.
- If platelet count is > 400 × 109/L, do not dose. Continue to assess the platelet count weekly. After the platelet count has fallen to < 200 × 109/L, resume Nplate at a dose reduced by 1 mcg/kg.
For Pediatric Patients with ITP
The initial dose of Nplate is 1 mcg/kg. Actual body weight at initiation of treatment should always be used when calculating initial dose. In pediatric patients, future dose adjustments are based on changes in platelet counts and changes in body weight. Reassessment of body weight is recommended every 12 weeks.
Adjust the weekly dose of Nplate by increments of 1 mcg/kg until the patient achieves a platelet count ≥ 50 × 109/L as necessary to reduce the risk for bleeding; do not exceed a maximum weekly dose of 10 mcg/kg. In a pediatric placebo-controlled clinical study, the median of the most frequent dose of Nplate received by patients during weeks 17 through 24 was 5.5 mcg/kg.
Adjust the dose as follows for pediatric patients:
- If the platelet count is < 50 × 109/L, increase the dose by 1 mcg/kg.
- If platelet count is > 200 × 109/L and ≤ 400 × 109/L for 2 consecutive weeks, reduce the dose by 1 mcg/kg.
- If platelet count is > 400 × 109/L, do not dose. Continue to assess the platelet count weekly. After the platelet count has fallen to < 200 × 109/L, resume Nplate at a dose reduced by 1 mcg/kg.
Patients with Hematopoietic Syndrome of Acute Radiation Syndrome
For Adult and Pediatric Patients (including term neonates)
The recommended dose of Nplate is 10 mcg/kg administered once as a subcutaneous injection. Administer the dose as soon as possible after suspected or confirmed exposure to radiation levels greater than 2 gray (Gy).
Administer Nplate regardless of whether a complete blood count (CBC) can be obtained. Estimate a patient's absorbed whole body radiation dose (i.e., level of radiation exposure) based on information from public health authorities, biodosimetry if available, or clinical findings such as time to onset of vomiting or lymphocyte depletion kinetics.
Preparation and Administration
To mitigate against medication errors (both overdose and underdose), ensure that these preparation and administration instructions are followed. Use aseptic technique. Only administer subcutaneously.
Nplate is supplied in single-dose vials as a sterile, preservative-free, white lyophilized powder that must be reconstituted as outlined in Table 1 and administered using a syringe with 0.01 mL graduations.
Calculation of Patient Dose
Multiply the patient’s weight (kg) by the prescribed dose to obtain the Calculated Patient Dose.
| Calculated Patient Dose (mcg) = Weight (kg) × Prescribed dose (mcg/kg) |
Reconstitution and Dilution of Nplate Single-Dose Vials
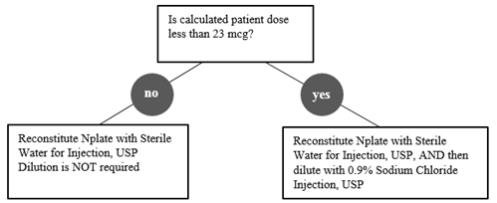
Reconstitute Nplate with Sterile Water for Injection, USP. Do not reconstitute or dilute with Bacteriostatic Water for Injection, USP or dilute with Bacteriostatic Sodium Chloride Injection, USP. If the Calculated Patient Dose is less than 23 mcg, dilution with 0.9% Sodium Chloride Injection, USP is required to reduce the concentration of Nplate (see Table 1). This reduced concentration allows for low-doses to be accurately calculated, and consistently measured with a 0.01 mL graduated syringe.
| Calculated Patient Dose | Strength* | Reconstitute with Sterile Water† | Dilute with Normal Saline‡ | Final Concentration |
|---|---|---|---|---|
| Calculated Dose greater than or equal to 23 mcg | 125 mcg | 0.44 mL | Not Required | 500 mcg/mL |
| 250 mcg | 0.72 mL | Not Required | ||
| 500 mcg | 1.2 mL | Not Required | ||
| Calculated Dose less than 23 mcg | 125 mcg | 0.44 mL | 1.38 mL | 125 mcg/mL |
| 250 mcg | 0.72 mL | 2.25 mL | ||
| 500 mcg | 1.2 mL | 3.75 mL | ||
Gently swirl and invert the vial to reconstitute. Avoid excess or vigorous agitation: DO NOT SHAKE. Generally, dissolution of Nplate takes less than 2 minutes. The reconstituted Nplate solution should be clear and colorless. Visually inspect the reconstituted solution for particulate matter and/or discoloration. Do not administer Nplate if particulate matter and/or discoloration is observed.
Calculate Volume to Administer by dividing the Calculated Patient Dose (mcg) by the final concentration of prepared solution. See Table 2 for final concentrations.
| Calculated Patient Dose | Final Concentration | Volume to Administer (mL) |
| Calculated Dose greater than or equal to 23 mcg | 500 mcg/mL | = Calculated Patient Dose / 500 mcg/mL |
| Calculated Dose less than 23 mcg | 125 mcg/mL | = Calculated Patient Dose / 125 mcg/mL |
Administration of Prepared Nplate Solution
Administer Nplate only using a syringe with 0.01 mL graduations for accurate dosage. Round volume to the nearest hundredth mL. Verify that the syringe contains the correct dosage.
Discard any unused portion. Do not pool unused portions from the vials. Do not administer more than one dose from a vial.
Storage of Reconstituted Solution
Reconstituted product with Sterile Water for Injection, USP that has not been further diluted can remain in the original vial at room temperature 25°C (77°F) or be refrigerated at 2°C to 8°C (36°F to 46°F) for up to 24 hours following reconstitution. Reconstituted product with Sterile Water for Injection, USP may be held in a syringe at room temperature 25°C (77°F) for a maximum of 4 hours following reconstitution. Protect product from light. Do not shake.
Storage of Diluted solution (after initial reconstitution)
Reconstituted and further diluted product with 0.9% Sodium Chloride Injection, USP can be held in a syringe at room temperature 25°C (77°F) or in the original vial refrigerated at 2°C to 8°C (36°F to 46°F) for no longer than 4 hours prior to administration. Protect product from light. Do not shake.
Frequently asked questions
- How long does Nplate (romiplostim) take to work?
- How is Nplate (romiplostim) administered?
- Is Nplate a chemotherapy drug?
- What is Nplate used for and how does it work?
- Does Nplate cause hair loss?
More about Nplate (romiplostim)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (11)
- Drug images
- Side effects
- Patient tips
- During pregnancy
- FDA approval history
- Drug class: platelet-stimulating agents
- Breastfeeding
- En español
Patient resources
Professional resources
Related treatment guides
See also:
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.