Urolet MB: Package Insert / Prescribing Info
Package insert / product label
Generic name: methenamine, sodium phosphate, monobasic, methylene blue, and hyoscyamine sulfate
Dosage form: tablet
Drug class: Urinary antispasmodics
Medically reviewed by Drugs.com. Last updated on Mar 25, 2025.
The Urolet MB brand name has been discontinued in the U.S. If generic versions of this product have been approved by the FDA, there may be generic equivalents available.
On This Page
| Methenamine, USP | 81.6 mg | |
| Monobasic Sodium Phosphate, USP | 40.8 mg | |
| Methylene Blue, USP | 10.8 mg | |
| Hyoscyamine Sulfate, USP | 0.12 mg |
Microcrystalline Cellulose, Mannitol, Croscarmellose Sodium, Magnesium Stearate, FD&C Blue #1.
METHENAMINE. [100-97-0] 1,3,5,7-Tetraazatricyclo [3.3.1.-13,7] decane; hexamethylenetetramine; HMT; HMTA; hexamine; 1,3,5,7-tetraazaadamantane hexamethylenemine; Uritone; Urotropin. C6H12N4; mol wt 140.19; C 51.40%, H 8.63%, N 39.96%. Methenamine (hexamethylenetetramine) exists as colorless, lustrous crystals or white crystalline powder. Its solutions are alkaline to litmus. Freely soluble in water, soluble in alcohol and in chloroform.
MONOBASIC SODIUM PHOSPHATE [7558-80-7] Phosphoric acid sodium salt (1:1); Sodium biphosphate; sodium dihydrogen phosphate; acid sodium phosphate; monosodium orthophosphate; primary sodium phosphate; H2NaO4P; mol wt 119.98, H 1.68%, Na 19.16%, O 53.34%, P 25.82%. Monohydrate, white, odorless slightly deliquesce crystals or granules. At 100° C loses all its water; when ignited it converts to metaphosphate. It is freely soluble in water and practically insoluble in alcohol. The aqueous solution is acid. pH of 0.1 molar aqueous solution at 25° C: 4.5.
HYOSCYAMINE SULFATE. [620-61-1] [3(S)-endo]-α-(Hydroxymethyl)-benzeneacetic acid 8-methyl- 8-azabicyclo[3.2.1]oct-3-yl ester sulfate(2:1)(salt); 1αH,5αH-tropan-3α-ol(-)-tropate (ester) sulfate(2:1)(salt); 3α-tropanyl S-(-)-tropate; I-tropic acid ester with tropine; I-tropine tropate. C34H48N2O10S. Hyoscyamine Sulfate is an alkaloid of belladonna. Exists as a white crystalline powder. Its solutions are alkaline to litmus. Affected by light, it is slightly soluble in water; freely soluble in alcohol; sparingly soluble in ether.
METHYLENE BLUE. [61-73-4] 3,7-Bis(dimethylamino) phenothiazin-5-ium chloride; C.I. Basic Blue 9; methylthioninium chloride; tetramethylthionine chloride; 3,7-bis(dimethylamino) phenazathionium chloride. C16H18ClN3S; mol wt 319.85, C 60.08%, H 5.67%, Cl 11.08%, N 13.14%, S 10.03%. Methylene Blue (Methylthionine chloride) exists as dark green crystals. It is soluble in water and in chloroform; sparingly soluble in alcohol.
Urolet MB - Clinical Pharmacology
METHENAMINE degrades in an acidic urine environment releasing formaldehyde which provides bactericidal or bacteriostatic action. It is well absorbed from the gastrointestinal tract. 70 to 90% reaches the urine unchanged at which point it is hydrolyzed if the urine is acidic. Within 24 hours it is almost completely (90%) excreted; of this amount at pH 5, approximately 20% is formaldehyde. Protein binding - some formaldehyde is bound to substances in the urine and surrounding tissues. Methenamine is freely distributed to body tissue and fluids but is not clinically significant as it does not hydrolyze at a pH greater than 6.8.
MONOBASIC SODIUM PHOSPHATE an acidifier, helps to maintain an acid pH in the urine necessary for the degradation of methenamine.
METHYLENE BLUE possesses weak antiseptic properties. It is well absorbed by the gastrointestinal tract and is rapidly reduced to leukomethylene blue which is stabilized in some combination form in the urine. 75% is excreted unchanged.
HYOSCYAMINE SULFATE is a parasympatholytic drug which relaxes smooth muscles and thus produces an antispasmodic effect. It is well absorbed from the gastrointestinal tract and is rapidly distributed throughout the body tissues. Most is excreted in the urine within 12 hours, 13% to 50% being unchanged. Protein binding for hyoscyamine sulfate is moderate and biotransformation is hepatic.
Indications and Usage for Urolet MB
UROLET MB™ is indicated for the treatment of symptoms of irritative voiding. Indicated for the relief of local symptoms, such as hypermotility which accompany lower urinary tract infections and antispasmodic. Indicated for the relief of nary tract symptoms caused by diagnostic procedures.
Contraindications
UROLET MB™ is contraindicated in patients with a hypersensitivity to any of the ingredients. Risk- benefit should be considered when the following medical problems exist: Cardiac disease (especially cardiac arrhythmias, congestive heart failure, coronary heart disease, and mitral stenosis); gastrointestinal tract obstructive disease; glaucoma; myasthenia gravis; acute urinary retention may be precipitated in obstructive uropathy (such as bladder neck obstruction due to prostatic hypertrophy).
Warnings
Do not exceed recommended dosage. If rapid pulse, dizziness, or blurring of vision occurs, discontinue use immediately.
Patients should be advised that urine will be colored blue when taking this medication. Do not exceed recommended dosage.
Precautions
Cross sensitivity and/or related problems
Patients intolerant of other belladonna alkaloids may be intolerant of this medication also. Delay in gastric emptying could complicate the management of gastric ulcers.
Drug Interactions:
Although the exact mechanism of this drug interaction is unknown, methylene blue inhibits the action of monoamine oxidase A— an enzyme responsible for breaking down serotonin in the brain. It is believed that when methylene blue is given to patients taking serotonergic psychiatric medications, high levels of serotonin can build up in the brain, causing toxicity. This is referred to as Serotonin Syndrome. Signs and symptoms of Serotonin Syndrome include mental changes (confusion, hyperactivity, memory problems), muscle twitching, excessive sweating, shivering or shaking, diarrhea, trouble with coordination, and/or fever.
Additional Information for Healthcare Professionals:
Methylene blue can interact with serotonergic psychiatric medications and cause serious CNS toxicity.
In emergency situations requiring life-threatening or urgent treatment with methylene blue (as described above), the availability of alternative interventions should be considered and the benefit of methylene blue treatment should be weighed against the risk of serotonin toxicity. If methylene blue must be administered to a patient receiving a serotonergic drug, the serotonergic drug must be immediately stopped, and the patient should be closely monitored for emergent symptoms of CNS toxicity for two weeks (five weeks if fluoxetine [Prozac] was taken), or until 24 hours after the last dose of methylene blue, whichever comes first.
In non-emergency situations when non-urgent treatment with methylene blue is contemplated and planned, the serotonergic psychiatric medication should be stopped to allow its activity in the brain to dissipate. Most serotonergic psychiatric drugs should be stopped at least 2 weeks in advance of methylene blue treatment. Fluoxetine (Prozac), which has a longer half-life compared to similar drugs, should be stopped at least 5 weeks in advance.
Treatment with the serotonergic psychiatric medication may be resumed 24 hours after the last dose of methylene blue.
Serotonergic psychiatric medications should not be started in a patient receiving methylene blue. Wait until 24 hours after the last dose of methylene blue before starting the antidepressant.
Educate your patients to recognize the symptoms of serotonin toxicity or CNS toxicity and advise them to contact a healthcare professional immediately if they experience any symptoms while taking serotonergic psychiatric medications or methylene blue.
As a result of hyoscyamine's effects on gastrointestinal motility and gastric emptying, absorption of other oral medications may be decreased during concurrent use with this combination medication.
Urinary alkalizers and thiazide diuretics:
May cause the urine to become alkaline reducing the effectiveness of methenamine by inhibiting its conversion to formaldehyde.
Antimuscarinics:
Concurrent use may intensify antimuscarinic effects of hyoscyamine because of secondary antimuscarinic activities of these medications.
Antacids/antidiarrheals:
Concurrent use may reduce absorption of hyoscyamine resulting in decreased therapeutic effectiveness. Concurrent use with antacids may cause urine to become alkaline reducing the effectiveness of methenamine by inhibiting its conversion to formaldehyde. Doses of these medications should be spaced 1 hour apart from doses of hyoscyamine.
Antimyasthenics:
Concurrent use with hyoscyamine may further reduce intestinal motility, therefore, caution is recommended.
Ketoconazole and hyoscyamine may cause increased gastrointestinal pH. Concurrent administration with hyoscyamine may result in marked reduction in the absorption of ketoconazole. Patients should be advised to take this combination at least 2 hours after ketoconazole.
Monoamine oxidase (MAO) inhibitors:
Concurrent use with hyoscyamine may intensify antimuscarinic side effects.
Opioid (narcotic) analgesics may result in increased risk of severe constipation.
Sulfonamides:
These drugs may precipitate with formaldehyde in the urine increasing the danger of crystalluria.
Patients should be advised that the urine and/or stools may become blue to blue-green as a result of the excretion of methylene blue.
Pregnancy/Reproduction (FDA Pregnancy Category C):
Hyoscyamine and methenamine cross the placenta. Studies have not been done in either animals or humans. It is not known whether UROLET MB™ can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity.
UROLET MB™ should be given to a pregnant woman only if clearly needed.
Nursing mothers:
Methenamine and traces of hyoscyamine are excreted in breast milk. Caution should be exercised when UROLET MB™ is administered to a nursing mother.
Prolonged use:
There have been no studies to establish the safety of prolonged use in humans. No known long-term animal studies have been performed to evaluate carcinogenic potential.
Adverse Reactions/Side Effects
Cardiovascular - rapid pulse, flushing
Central Nervous System - blurred vision, dizziness, drowsiness
Respiratory - shortness of breath or troubled breathing
Genitourinary - difficult micturition, acute urinary retention
Gastrointestinal - dry mouth, nausea and vomiting
Serious allergic reactions to this drug are rare. Seek immediate medical attention if you notice symptoms of a serious allergic reaction, including itching, rash, severe dizziness, swelling or trouble breathing.
This medication can cause urine and sometimes stools to turn blue to blue-green. This effect is harmless and will subside after medication is stopped.
Call your doctor or physician for medical advice about side effects. To report SUSPECTED ADVERSE REACTIONS, contact Burel Pharmaceuticals, Inc at 1-601-706-9819 or FDA at 1-800-FDA-1088, www.fda.gov/medwatch.
Related/similar drugs
Drug Abuse and Dependence
A dependence on the use of UROLET MB™ has not been reported and due to the nature of its ingredients, abuse of UROLET MB™ is not expected.
Overdosage
Emesis or gastric lavage. Slow intravenous administration of physostigmine in doses of 1 to 4 mg (0.5 to 1 mg in children) repeated as needed in one to two hours to reverse severe antimuscarinic symptoms.
Administration of small doses of diazepam to control excitement and seizures. Artificial respiration with oxygen if needed for respiratory depression. Adequate hydration.
Symptomatic treatment as necessary.
If overdose is suspected, contact the poison control center at 1-800-222-1222, or your local emergency room immediately.
How is Urolet MB supplied
UROLET MB™ tablets are light blue to blue, biconvex, debossed with “BL 04” with scoreline and plain on the other side, available in bottles of 100 tablets, NDC 35573-302-100 and bottle of 30 tablets, NDC 35573-302-30.
STORAGE
Store in a cool, dry place at controlled room temperature 15° to 30°C (59° to 86°F). Keep container tightly closed. Protect from moisture and direct sunlight.
Dispense in a tight, light-resistant container as defined in the USP/NF with a child resistant closure.
KEEP OUT OF THE REACH OF CHILDREN.
IN CASE OF ACCIDENTAL OVERDOSE, SEEK PROFESSIONAL ASSISTANCE OR CONTACT A POISON CONTROL CENTER IMMEDIATELY.
Note: Patients should be advised that urine will be colored blue when taking this medication.
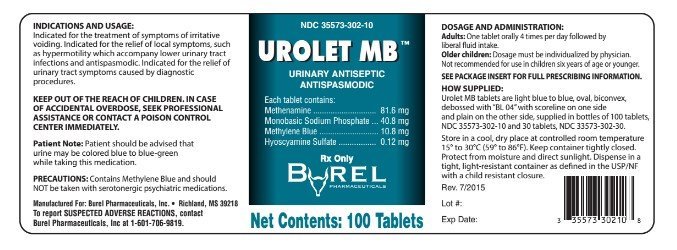
PRINCIPAL DISPLAY PANEL - 100 count Tablet Bottle Label
NDC 35573-302-100
UROLET MB™
URINARY ANTISEPTIC
ANTISPASMODIC
DESCRIPTION: Each tablet contains:
| Methenamine, USP | 81.6 mg |
| Monobasic Sodium Phosphate, USP | 40.8 mg |
| Methylene Blue, USP | 10.8 mg |
| Hyoscyamine Sulfate, USP | 0.12 mg |
CONTENTS: 100 TABLETS
RX ONLY
Manufactured for:
Burel Pharmaceuticals, Inc
Richland, MS 39218
PRINCIPAL DISPLAY PANEL - 100 count Tablet Bottle Label
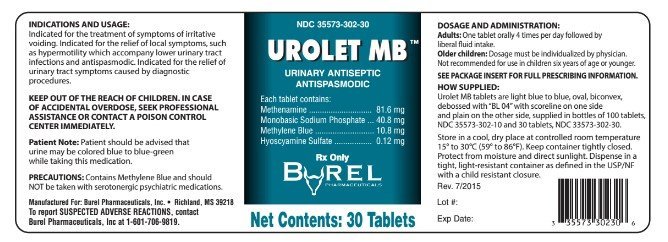
NDC 35573-302-30
UROLET MB™
URINARY ANTISEPTIC
ANTISPASMODIC
DESCRIPTION: Each tablet contains:
| Methenamine, USP | 81.6 mg |
| Monobasic Sodium Phosphate, USP | 40.8 mg |
| Methylene Blue, USP | 10.8 mg |
| Hyoscyamine Sulfate, USP | 0.12 mg |
CONTENTS: 30 TABLETS
RX ONLY
Manufactured for:
Burel Pharmaceuticals, Inc
Richland, MS 39218
| UROLET MB
methenamine, sodium phosphate, monobasic, methylene blue, and hyoscyamine sulfate tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Burel Pharmaceuticals, Inc (002152814) |
More about Urolet MB (hyoscyamine / methenamine / methylene blue / sodium biphosphate)
- Check interactions
- Compare alternatives
- Imprints, shape & color data
- Side effects
- Dosage information
- Drug class: urinary antispasmodics
