Thyroshield: Package Insert / Prescribing Info
Package insert / product label
Generic name: potassium iodide
Dosage form: oral solution
Drug classes: Antithyroid agents, Expectorants
Medically reviewed by Drugs.com. Last updated on Mar 25, 2025.
Indications and Usage for Thyroshield
Helps prevent radioactive iodine from getting into the thyroid gland during a nuclear radiation emergency. Use along with other emergency measures recommended by public officials.
Warnings
Allergy alert: Iodine may cause an allergic reaction with 1 or more of the following symptoms:
- shortness of breath or wheezing
- skin rash
- trouble breathing, speaking or swallowing
- swelling
- fever and joint pain
Do not use if you have ever had
- an allergic reaction to iodine
- nodular thyroid disease with heart disease
- hypocomplementemic vasculitis
- dermatitis herpetiformis
Stop use and ask a doctor if you have
- an allergic reaction. Get medical help right away if you have trouble breathing, speaking or swallowing; shortness of breath; wheezing; swelling of the mouth, tongue or throat; or rash.
- irregular heart or chest pain. Get medical help right away.
- swelling of the hands or feet, fever, or joint pain.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Thyroshield Dosage and Administration
- use as directed by public officials in the event of a nuclear radiation emergency.
- do not take more than 1 dose in 24 hours.
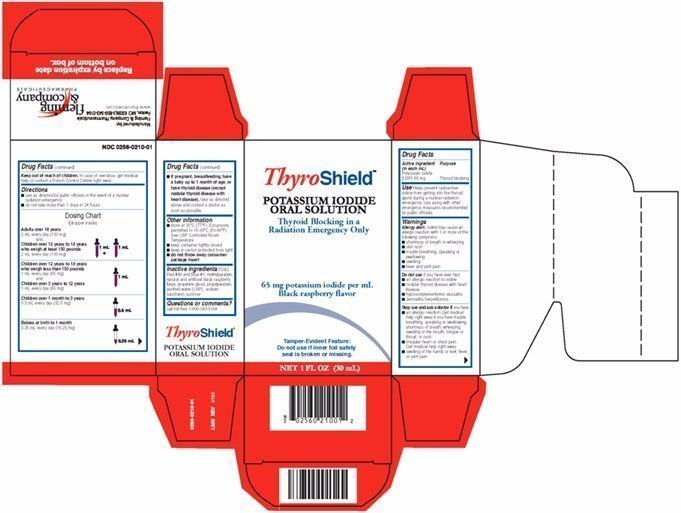
Dosing Chart
(dropper inside)
Adults over 18 years
2 mL every day (130 mg)
and
Children over 12 years to 18 years
who weigh at least 150 pounds
2 mL every day (130 mg)
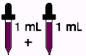
Children over 12 years to 18 years
who weigh less than 150 pounds
1 mL every day (65 mg)
and
Children over 3 years to 12 years
1 mL every day (65 mg)

Children over 1 month to 3 years
0.5 mL every day (32.5 mg)

Babies at birth to 1 month
0.25 mL every day (16.25 mg)

- If pregnant, breastfeeding, have a baby up to 1 month of age or have thyroid disease (except nodular thyroid disease with heart disease), take as directed above and contact a doctor as soon as possible.
Other Information
- store at 25°C (77°F). Excursions permitted to 15-30°C (59-86°F). See USP Controlled Room Temperature.
- keep container tightly closed
- keep in carton protected from light
- do not throw away consumer package insert
Inactive ingredients
FD&C Red #40 and Blue #1, methylparaben, natural and artificial black raspberry flavor, propylene glycol, propylparaben, purified water (USP), sodium saccharin, sucrose
Patient Information
ThyroShield®
(Potassium Iodide Oral Solution, 1 mL = 65 mg)
(Abbreviated KI)
Take potassium iodide (KI) only when public officials tell you. In a nuclear radiation emergency, radioactive iodine could be released into the air. KI protects only the thyroid gland from uptake of radioactive iodine. Therefore, KI should be used along with other emergency measures that will be recommended to you by public officials.
If you are told to take this medicine, take it one time every 24 hours. Do not take it more often. More KI will not help you. Too much KI may increase the chances of side effects. Do not take this medicine if you know you are allergic to iodine (see SIDE EFFECTS below).
Related/similar drugs
Thyroshield Description
Each milliliter (1 mL) of ThyroShield® contains 65 mg of potassium iodide (USP) in a black raspberry-flavored solution. Inactive ingredients are: FD&C Red #40 and Blue #1, methylparaben, natural and artificial black raspberry flavor, propylene glycol, propylparaben, purified water (USP), sodium saccharin, sucrose.
Indications and Usage for Thyroshield
ThyroShield® (Potassium Iodide Oral Solution) is a thyroid blocking medicine that is used in a nuclear radiation emergency only.
DIRECTIONS FOR USE:
Use only as directed by public officials if a nuclear radiation emergency happens.
Dose:
Adults over 18 years 2 mL every day (130 mg)
Children over 12 years to 18 years 2 mL every day (130 mg)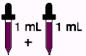
who weigh at least 150 pounds
Children over 12 years to 18 years 1 mL every day (65 mg)
who weigh less than 150 pounds
Children over 3 years to 12 years 1 mL every day (65 mg)
Children over 1 month to 3 years 0.5 mL every day (32.5 mg)
Babies at birth to 1 month 0.25 mL every day (16.25 mg)
Take KI every day (every 24 hours) as directed by public officials. Do not take more than 1 dose in 24 hours. More will not help you. Too much medicine may increase the chances of side effects.
Pregnant or breastfeeding women, or babies under 1 month of age: Take as directed above and call a doctor as soon as possible. Repeat dosing should be avoided. It is recommended that thyroid function be checked in babies less than 1 month of age that take KI. Women who are pregnant or breastfeeding should also be checked by a doctor if repeat dosing is necessary. Although these precautions should be taken, the benefits of short-term use of KI to block uptake of radioactive iodine by the thyroid gland far exceed its chances of side effects.
Patients with thyroid disease: If you have both a nodular thyroid condition such as multinodular goiter with heart disease, you should not take KI. Patients with other thyroid conditions may take KI as directed above, but call a doctor if you need to take KI for more than a few days.
Warnings
People who are allergic to iodine, have dermatitis herpetiformis or hypocomplementemic vasculitis, or have nodular thyroid disease with heart disease should not take KI. Keep out of the reach of children. In case of an allergic reaction (difficulty breathing, speaking or swallowing; wheezing; shortness of breath or swelling of the mouth or throat), call 911 or get medical care right away. In case of overdose, get medical help or call a Poison Control Center right away.
HOW POTASSIUM IODIDE WORKS:
Certain forms of iodine help your thyroid gland work right. Most people get the iodine they need from foods like iodized salt or fish. The thyroid can “store” or hold only a certain amount of iodine.
In nuclear radiation emergency, radioactive iodine may be released in the air. This material may be breathed or swallowed. It may enter the thyroid gland and damage it. The damage would probably not show itself for years. Children are most likely to have thyroid damage.
If you take KI, it will block or reduce the chances that radioactive iodine will enter your thyroid gland.
WHO SHOULD NOT TAKE POTASSIUM IODIDE:
People should avoid KI if they are allergic to iodine, have dermatitis herpetiformis or hypocomplementemic vasculitis, or have nodular thyroid disease with heart disease, because these conditions may increase the chances of side effects to iodine.
HOW AND WHEN TO TAKE POTASSIUM IODIDE:
KI should be taken as soon as possible after public officials tell you. If you are told to repeat the dose, you should take the second dose 24 hours after the first dose. Do not take it sooner. More KI will not help you because the thyroid can “hold” only certain amounts of iodine. Taking more than 1 dose per day will increase the chances of side effects. The public officials will tell you how many days to take KI. You should take KI until the chances of major exposure to radioactive iodine by breathing or swallowing stops.
SIDE EFFECTS:
Short-term use of KI at the recommended dose is safe. You should not take this drug for longer than you are told.
Possible side effects include: swelling of the salivary glands, nausea, vomiting, diarrhea, stomach ache, fever, headache, metallic taste, and allergic reactions. Allergic reaction can include
- skin rashes such as hives
- swelling of various parts of the body such as the face, lips, tongue, throat, hands or feet
- fever with joint pain
- trouble breathing, speaking or swallowing
- wheezing or shortness of breath
Get medical attention right away if you have trouble breathing, speaking or swallowing; wheezing; shortness of breath; or swelling of the mouth, tongue or throat.
Taking iodide, in rare cases, may cause overactivity of the thyroid gland, underactivity of the thyroid gland, or enlargement of the thyroid gland (goiter). Symptoms of an overactive thyroid gland may include an irregular heart beat and chest pain. Patients with thyroid disease are more likely to get these side effects. Babies under 1 month of age are more likely to get an underactive thyroid gland (hypothyroidism).
INACTIVE INGREDIENTS:
FD&C Red #40 and Blue #1, methylparaben, natural and artificial black raspberry flavor, propylene glycol, propylparaben, purified water (USP), sodium saccharin, sucrose.
HOW SUPPLIED:
ThyroShield® (Potassium Iodide Oral Solution) is supplied in 1 oz (30 mL) bottles. Each mL contains 65 mg potassium iodide. Store at 25º C (77º F). Excursions permitted to 15-30º C (59-86º F). [See USP Controlled Room Temperature.] Keep container tightly closed and in carton, protected from light.
Manufactured by Fleming & Company, Pharmaceuticals, Fenton, St. Louis Co., MO 63026
1-800-343-0164.
L240 Rev. 0207
| THYROSHIELD
potassium iodide solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Fleming & Company, Pharmaceuticals (006491351) |
More about ThyroShield (potassium iodide)
- Check interactions
- Compare alternatives
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- Drug class: antithyroid agents