Onglyza: Package Insert / Prescribing Info
Package insert / product label
Generic name: saxagliptin
Dosage form: tablet, film coated
Drug class: Dipeptidyl peptidase 4 inhibitors
Medically reviewed by Drugs.com. Last updated on Oct 22, 2024.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Drug Interactions
- Use In Specific Populations
- Overdosage
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Storage and Handling
- Patient Counseling Information
- Medication Guide
Highlights of Prescribing Information
ONGLYZA® (saxagliptin) tablets, for oral use
Initial U.S. Approval: 2009
Indications and Usage for Onglyza
Onglyza Dosage and Administration
- •
- Recommended dosage is 2.5 mg or 5 mg orally once daily taken regardless of meals. (2.1)
- •
- Patients with an eGFR <45 mL/min/1.73 m2 (moderate or severe renal impairment, or end-stage renal disease): Recommended dosage is 2.5 mg once daily regardless of meals. (2.2)
- •
- Assess renal function before starting ONGLYZA and periodically thereafter. (2.2)
- •
- Limit the dosage of ONGLYZA to 2.5 mg daily for patients also taking strong cytochrome P450 3A4/5 (CYP3A4/5) inhibitors (e.g., ketoconazole). (2.3)
Dosage Forms and Strengths
- •
- Tablets: 5 mg and 2.5 mg. (3)
Contraindications
- •
- History of a serious hypersensitivity reaction (e.g., anaphylaxis, angioedema, exfoliative skin conditions) to saxagliptin or any of the ingredients in ONGLYZA. (4)
Warnings and Precautions
- •
- Pancreatitis: There have been postmarketing reports of acute pancreatitis. If pancreatitis is suspected, promptly discontinue ONGLYZA. (5.1)
- •
- Heart Failure: Consider the risks and benefits of ONGLYZA in patients who have known risk factors for heart failure. Monitor patients for signs and symptoms. (5.2)
- •
- Hypoglycemia with Concomitant Use of Insulin or Insulin Secretagogues: Consider a lower dosage of insulin or insulin secretagogue when used in combination wtih ONGLYZA. (5.3)
- •
- Hypersensitivity-Related Events There have been postmarketing reports of serious hypersensitivity reactions such as anaphylaxis, angioedema, and exfoliative skin conditions. If hypersensitivity reactions occur, discontinue ONGLYZA, treat promptly, and monitor until signs and symptoms resolve. (5.4)
- •
- Arthralgia: Severe and disabling arthralgia has been reported in patients taking DPP-4 inhibitors. Consider as a possible cause for severe joint pain and discontinue drug if appropriate. (5.5)
- •
- Bullous Pemphigoid: There have been postmarketing reports of bullous pemphigoid requiring hospitalization in patients taking DPP-4 inhibitors. Tell patients to report development of blisters or erosions. If bullous pemphigoid is suspected, discontinue ONGLYZA. (5.6)
Adverse Reactions/Side Effects
- •
- Most common adverse reactions (incidence ≥5% and more often than placebo) are upper respiratory tract infection, urinary tract infection, and headache. (6.1)
- •
- Peripheral edema was reported more commonly in patients treated with the combination of ONGLYZA and a thiazolidinedione (TZD) than in patients treated with the combination of placebo and TZD. (6.1)
- To report SUSPECTED ADVERSE REACTIONS, contact AstraZeneca at 1-800-236-9933 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- •
- Strong CYP3A4/5 inhibitors (e.g., ketoconazole): Coadministration with ONGLYZA significantly increases saxagliptin concentrations. Limit ONGLYZA dosage to 2.5 mg once daily when coadministered with a strong CYP3A4/5 inhibitor. (7.1)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 10/2024
Full Prescribing Information
1. Indications and Usage for Onglyza
1.1 Monotherapy and Combination Therapy
ONGLYZA is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus [see Clinical Studies (14)].
2. Onglyza Dosage and Administration
2.1 Recommended Dosage
The recommended dosage of ONGLYZA is 2.5 mg or 5 mg orally once daily taken regardless of meals. Do not cut, crush, or chew ONGLYZA tablets.
If a dose is missed, advise patients not to take an extra dose. Resume treatment with the next dose.
2.2 Dosage in Patients with Renal Impairment
Assess renal function prior to initiation of ONGLYZA and then as clinically indicated [see Use in Specific Populations (8.6)].
The recommended dosage of ONGLYZA in patients with an eGFR greater than or equal to 45 mL/minute/1.73 m2 is the same as the recommended dosage in patients with normal renal function [see Dosage and Administration (2.1)].
The dosage of ONGLYZA is 2.5 mg orally once daily for patients with eGFR <45 mL/min/1.73 m2 [which includes a subset of moderate or severe renal impairment, or with end-stage renal disease (ESRD) requiring hemodialysis] [see Clinical Pharmacology (12.3) and Clinical Studies (14.2)]. ONGLYZA should be administered following hemodialysis. ONGLYZA has not been studied in patients undergoing peritoneal dialysis.
2.3 Dosage Modification with Concomitant Use of Strong CYP3A4/5 Inhibitors
The dosage of ONGLYZA is 2.5 mg orally once daily when used concomitantly with strong cytochrome P450 3A4/5 (CYP3A4/5) inhibitors (e.g., ketoconazole, atazanavir, clarithromycin, indinavir, itraconazole, nefazodone, nelfinavir, ritonavir, saquinavir, and telithromycin) [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)].
3. Dosage Forms and Strengths
Tablets:
- •
- 5 mg: pink, biconvex, round, film-coated tablets with “5” printed on one side and “4215” printed on the reverse side, in blue ink.
- •
- 2.5 mg: pale yellow to light yellow, biconvex, round, film-coated tablets with “2.5” printed on one side and “4214” printed on the reverse side, in blue ink.
4. Contraindications
ONGLYZA is contraindicated in patients with a history of a serious hypersensitivity reaction to saxagliptin or any of the ingredients in ONGLYZA. Reactions such as anaphylaxis, angioedema, or exfoliative skin conditions have been reported with ONGLYZA [see Warnings and Precautions (5.4) and Adverse Reactions (6.2)].
5. Warnings and Precautions
5.1 Pancreatitis
There have been postmarketing reports of acute pancreatitis in patients taking ONGLYZA. In a cardiovascular outcomes trial enrolling participants with established atherosclerotic cardiovascular disease (ASCVD) or multiple risk factors for ASCVD (SAVOR trial), cases of definite acute pancreatitis were confirmed in 17 of 8240 (0.2%) patients receiving ONGLYZA compared to 9 of 8173 (0.1%) receiving placebo. Preexisting risk factors for pancreatitis were identified in 88% (15/17) of those patients receiving ONGLYZA and in 100% (9/9) of those patients receiving placebo.
After initiation of ONGLYZA, observe patients for signs and symptoms of pancreatitis. If pancreatitis is suspected, promptly discontinue ONGLYZA and initiate appropriate management. It is unknown whether patients with a history of pancreatitis are at increased risk for the development of pancreatitis while using ONGLYZA.
5.2 Heart Failure
In a cardiovascular outcomes trial enrolling participants with established ASCVD or multiple risk factors for ASCVD (SAVOR trial), more patients randomized to ONGLYZA (289/8280, 3.5%) were hospitalized for heart failure compared to patients randomized to placebo (228/8212, 2.8%). In a time-to-first-event analysis the risk of hospitalization for heart failure was higher in the ONGLYZA group (estimated Hazard Ratio: 1.27; 95% CI: 1.07, 1.51). Patients with a prior history of heart failure and patients with renal impairment had a higher risk for hospitalization for heart failure, irrespective of treatment assignment.
Consider the risks and benefits of ONGLYZA prior to initiating treatment in patients at a higher risk for heart failure. Observe patients for signs and symptoms of heart failure during therapy. Advise patients of the characteristic symptoms of heart failure and to immediately report such symptoms. If heart failure develops, evaluate and manage according to current standards of care and consider discontinuation of ONGLYZA.
5.3 Hypoglycemia with Concomitant Use of Insulin or Insulin Secretagogues
When ONGLYZA was used in combination with insulin or an insulin secretagogue, the incidence of confirmed hypoglycemia was increased over that of placebo used in combination with insulin or an insulin secretagogue [see Adverse Reactions (6.1)]. Therefore, a lower dosage of insulin or an insulin secretagogue may be required to reduce the risk of hypoglycemia when used in combination with ONGLYZA [see Drug Interactions (7.2)]. Inform patients using these concomitant medications of the risk of hypoglycemia and educate them on the signs and symptoms of hypoglycemia.
5.4 Hypersensitivity Reactions
There have been postmarketing reports of serious hypersensitivity reactions in patients treated with ONGLYZA. These reactions include anaphylaxis, angioedema, and exfoliative skin conditions. Onset of these reactions occurred within the first 3 months after initiation of treatment with ONGLYZA, with some reports occurring after the first dose. If a serious hypersensitivity reaction is suspected, discontinue ONGLYZA, assess for other potential causes for the event, and institute alternative treatment for diabetes [see Adverse Reactions (6.2)].
Use caution in a patient with a history of angioedema to another dipeptidyl peptidase-4 (DPP-4) inhibitor because it is unknown whether such patients will be predisposed to angioedema with ONGLYZA.
5.5 Severe and Disabling Arthralgia
There have been postmarketing reports of severe and disabling arthralgia in patients taking DPP-4 inhibitors. The time to onset of symptoms following initiation of drug therapy varied from one day to years. Patients experienced relief of symptoms upon discontinuation of the medication. A subset of patients experienced a recurrence of symptoms when restarting the same drug or a different DPP-4 inhibitor. Consider DPP-4 inhibitors as a possible cause for severe joint pain and discontinue drug if appropriate.
5.6 Bullous Pemphigoid
Postmarketing cases of bullous pemphigoid requiring hospitalization have been reported with DPP‑4 inhibitor use. In reported cases, patients typically recovered with topical or systemic immunosuppressive treatment and discontinuation of the DPP-4 inhibitor. Tell patients to report development of blisters or erosions while receiving ONGLYZA. If bullous pemphigoid is suspected, ONGLYZA should be discontinued and referral to a dermatologist should be considered for diagnosis and appropriate treatment.
6. Adverse Reactions/Side Effects
The following serious adverse reactions are described below or elsewhere in the prescribing information:
- •
- Pancreatitis [see Warnings and Precautions (5.1)]
- •
- Heart Failure [see Warnings and Precautions (5.2)]
- •
- Hypoglycemia with Concomitant Use of Insulin or Insulin Secretagogues [see Warnings and Precautions (5.3)]
- •
- Hypersensitivity Reactions [see Warnings and Precautions (5.4)]
- •
- Severe and disabling arthralgia [see Warnings and Precautions (5.5)]
- •
- Bullous pemphigoid [see Warnings and Precautions (5.6)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse Reactions in Placebo-Controlled Trials in Adults with Type 2 Diabetes Mellitus
The data in Table 1 are derived from a pool of 5 placebo-controlled clinical trials [see Clinical Studies (14)]. These data shown in the table reflect exposure of 882 patients to ONGLYZA and a mean duration of exposure to ONGLYZA of 21 weeks. The mean age of these patients was 55 years, 1.4 % were 75 years of age or older and 48.4% were male. The population was 67.5% White, 4.6% Black or African American, 17.4% Asian, 10.5% other races and 9.8% were of Hispanic or Latino ethnicity. At baseline the population had type 2 diabetes mellitus for an average of 5.2 years and a mean HbA1c of 8.2%. Baseline estimated renal function was normal or mildly impaired (eGFR≥60 mL/min/1.73 m2) in 91% of these patients.
Table 1 shows common adverse reactions, excluding hypoglycemia, associated with the use of ONGLYZA. These adverse reactions occurred more commonly on ONGLYZA than on placebo and occurred in at least 5% of patients treated with ONGLYZA.
| % of Patients | ||
|---|---|---|
| ONGLYZA 5 mg
N=882 | Placebo
N=799 |
|
|
||
|
Upper respiratory tract infection |
7.7 |
7.6 |
|
Urinary tract infection |
6.8 |
6.1 |
|
Headache |
6.5 |
5.9 |
In patients treated with ONGLYZA 2.5 mg, headache (6.5%) was the only adverse reaction reported at a rate ≥5% and more commonly than in patients treated with placebo.
In the add-on to TZD trial, the incidence of peripheral edema was higher for ONGLYZA 5 mg versus placebo (8.1% and 4.3%, respectively). The incidence of peripheral edema for ONGLYZA 2.5 mg was 3.1%. None of the reported adverse reactions of peripheral edema resulted in trial drug discontinuation. Rates of peripheral edema for ONGLYZA 2.5 mg and ONGLYZA 5 mg versus placebo were 3.6% and 2% versus 3% given as monotherapy, 2.1% and 2.1% versus 2.2% given as add-on therapy to metformin HCl, and 2.4% and 1.2% versus 2.2% given as add-on therapy to glyburide.
The incidence rate of fractures was 1.0 and 0.6 per 100 patient-years, respectively, for ONGLYZA (pooled analysis of 2.5 mg, 5 mg, and 10 mg) and placebo. The 10 mg dosage is not an approved dosage. The incidence rate of fracture events in patients who received ONGLYZA did not increase over time. Causality has not been established and nonclinical studies have not demonstrated adverse effects of ONGLYZA on bone.
An event of thrombocytopenia, consistent with a diagnosis of idiopathic thrombocytopenic purpura, was observed in the clinical program. The relationship of this event to ONGLYZA is not known.
Discontinuation of therapy due to adverse reactions occurred in 2.2%, 3.3%, and 1.8% of patients receiving ONGLYZA 2.5 mg, ONGLYZA 5 mg, and placebo, respectively. The most common adverse reactions (reported in at least 2 patients treated with ONGLYZA 2.5 mg or at least 2 patients treated with ONGLYZA 5 mg) associated with premature discontinuation of therapy included lymphopenia (0.1% and 0.5% versus 0%, respectively), rash (0.2% and 0.3% versus 0.3%), blood creatinine increased (0.3% and 0% versus 0%), and blood creatine phosphokinase increased (0.1% and 0.2% versus 0%).
Adverse Reactions with Concomitant Use with Insulin
In the add-on to insulin trial [see Clinical Studies (14.1)], the incidence of adverse reactions, including serious adverse reactions and discontinuations due to adverse reactions, was similar between ONGLYZA and placebo, except for confirmed hypoglycemia [see Adverse Reactions (6.1)].
Hypoglycemia
Adverse reactions of hypoglycemia were based on all reports of hypoglycemia. A concurrent glucose measurement was not required or was normal in some patients. Therefore, it is not possible to conclusively determine that all these reports reflect true hypoglycemia.
In the add-on to glyburide trial, the overall incidence of reported hypoglycemia was higher for ONGLYZA 2.5 mg and ONGLYZA 5 mg (13.3% and 14.6%) versus placebo (10.1%). The incidence of confirmed hypoglycemia in this trial, defined as symptoms of hypoglycemia accompanied by a fingerstick glucose value of ≤50 mg/dL, was 2.4% and 0.8% for ONGLYZA 2.5 mg and ONGLYZA 5 mg and 0.7% for placebo [see Warnings and Precautions (5.3)]. The incidence of reported hypoglycemia for ONGLYZA 2.5 mg and ONGLYZA 5 mg versus placebo given as monotherapy was 4% and 5.6% versus 4.1%, respectively, 7.8% and 5.8% versus 5% given as add-on therapy to metformin HCl, and 4.1% and 2.7% versus 3.8% given as add-on therapy to TZD. The incidence of reported hypoglycemia was 3.4% in treatment-naive patients given ONGLYZA 5 mg plus metformin HCl and 4% in patients given metformin HCl alone.
In the active-controlled trial comparing add-on therapy with ONGLYZA 5 mg to glipizide in patients inadequately controlled on metformin HCl alone, the incidence of reported hypoglycemia was 3% (19 events in 13 patients) with ONGLYZA 5 mg versus 36.3% (750 events in 156 patients) with glipizide. Confirmed symptomatic hypoglycemia (accompanying fingerstick blood glucose ≤50 mg/dL) was reported in none of the ONGLYZA-treated patients and in 35 glipizide-treated patients (8.1%) (p<0.0001).
In the add-on to insulin trial, the overall incidence of reported hypoglycemia was 18.4% for ONGLYZA 5 mg and 19.9% for placebo. However, the incidence of confirmed symptomatic hypoglycemia (accompanying fingerstick blood glucose ≤50 mg/dL) was higher with ONGLYZA 5 mg (5.3%) versus placebo (3.3%).
In the add-on to metformin HCl plus sulfonylurea trial, the overall incidence of reported hypoglycemia was 10.1% for ONGLYZA 5 mg and 6.3% for placebo. Confirmed hypoglycemia was reported in 1.6% of the ONGLYZA-treated patients and in none of the placebo-treated patients [see Warnings and Precautions (5.3)].
Hypersensitivity Reactions
Hypersensitivity reactions, such as urticaria and facial edema in the 5-trial pooled analysis up to Week 24 were reported in 1.5%, 1.5%, and 0.4% of patients who received ONGLYZA 2.5 mg, ONGLYZA 5 mg, and placebo, respectively. None of these events in patients who received ONGLYZA required hospitalization or were reported as life-threatening by the investigators. One ONGLYZA-treated patient in this pooled analysis discontinued due to generalized urticaria and facial edema.
Renal Impairment
In the SAVOR trial, adverse reactions related to renal impairment, including laboratory changes (i.e., doubling of serum creatinine compared with baseline and serum creatinine >6 mg/dL), were reported in 5.8% (483/8280) of ONGLYZA-treated patients and 5.1% (422/8212) of placebo-treated patients. The most frequently reported adverse reactions included renal impairment (2.1% vs. 1.9%), acute renal failure (1.4% vs. 1.2%), and renal failure (0.8% vs. 0.9%), in the ONGLYZA versus placebo groups, respectively. From baseline to the end of treatment, there was a mean decrease in eGFR of 2.5 mL/min/1.73 m2 for ONGLYZA-treated patients and a mean decrease of 2.4 mL/min/1.73 m2 for placebo-treated patients. More patients randomized to ONGLYZA (421/5227, 8.1%) compared to patients randomized to placebo (344/5073, 6.8%) had downward shifts in eGFR from >50 mL/min/1.73 m2 (i.e., normal or mild renal impairment) to ≤50 mL/min/1.73 m2 (i.e., moderate or severe renal impairment). The proportions of patients with renal adverse reactions increased with worsening baseline renal function and increased age, regardless of treatment assignment.
Infections
In the unblinded, controlled, clinical trial database for ONGLYZA to date, there have been 6 (0.12%) reports of tuberculosis among the 4959 ONGLYZA-treated patients (1.1 per 1000 patient-years) compared to no reports of tuberculosis among the 2868 comparator-treated patients. Two of these six cases were confirmed with laboratory testing. The remaining cases had limited information or had presumptive diagnoses of tuberculosis. None of the six cases occurred in the United States or in Western Europe. One case occurred in Canada in a patient originally from Indonesia who had recently visited Indonesia. The duration of treatment with ONGLYZA until report of tuberculosis ranged from 144 to 929 days. Post-treatment lymphocyte counts were consistently within the reference range for four cases. One patient had lymphopenia prior to initiation of ONGLYZA that remained stable throughout ONGLYZA treatment. The final patient had an isolated lymphocyte count below normal approximately four months prior to the report of tuberculosis. There have been no spontaneous reports of tuberculosis associated with ONGLYZA use. Causality has not been estimated and there are too few cases to date to determine whether tuberculosis is related to ONGLYZA use.
There has been one case of a potential opportunistic infection in the unblinded, controlled clinical trial database to date in an ONGLYZA-treated patient who developed suspected foodborne fatal salmonella sepsis after approximately 600 days of ONGLYZA therapy. There have been no spontaneous reports of opportunistic infections associated with ONGLYZA use.
Vital Signs
No clinically meaningful changes in vital signs have been observed in patients treated with ONGLYZA.
Laboratory Tests
Absolute Lymphocyte Counts
There was a dose-related mean decrease in absolute lymphocyte count observed with ONGLYZA. From a baseline mean absolute lymphocyte count of approximately 2200 cells/microL, mean decreases of approximately 100 and 120 cells/microL with ONGLYZA 5 mg and 10 mg, respectively, relative to placebo were observed at 24 weeks in a pooled analysis of five placebo-controlled clinical trials. Similar effects were observed when ONGLYZA 5 mg was given in initial combination with metformin HCl compared to metformin HCl alone. There was no difference observed for ONGLYZA 2.5 mg relative to placebo. The proportion of patients who were reported to have a lymphocyte count ≤750 cells/microL was 0.5%, 1.5%, 1.4%, and 0.4% in the ONGLYZA 2.5 mg, 5 mg, 10 mg, and placebo groups, respectively. In most patients, recurrence was not observed with repeated exposure to ONGLYZA although some patients had recurrent decreases upon rechallenge that led to discontinuation of ONGLYZA. The decreases in lymphocyte count were not associated with clinically relevant adverse reactions. The 10 mg dosage is not an approved dosage.
In the SAVOR trial mean decreases of approximately 84 cells/microL with ONGLYZA relative to placebo was observed. The proportion of patients who experienced a decrease in lymphocyte counts to a count of ≤750 cells/microL was 1.6% (136/8280) and 1.0% (78/8212) on ONGLYZA and placebo, respectively.
The clinical significance of this decrease in lymphocyte count relative to placebo is not known. When clinically indicated, such as in settings of unusual or prolonged infection, lymphocyte count should be measured. The effect of ONGLYZA on lymphocyte counts in patients with lymphocyte abnormalities (e.g., human immunodeficiency virus) is unknown.
6.2 Postmarketing Experience
Additional adverse reactions have been identified during post-approval use of ONGLYZA. Because these reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- •
- Gastrointestinal Disorders: Pancreatitis
- •
- Immune System Disorders: Hypersensitivity reactions including anaphylaxis, angioedema, and exfoliative skin conditions
- •
- Musculoskeletal and Connective Tissue Disorders: Rhabdomyolysis, Severe and disabling arthralgia
- •
- Skin and Subcutaneous Tissue Disorders: Bullous pemphigoid
Related/similar drugs
7. Drug Interactions
7.1 Strong Inhibitors of CYP3A4/5 Enzymes
Ketoconazole significantly increased saxagliptin exposure. Similar significant increases in plasma concentrations of saxagliptin are anticipated with other strong CYP3A4/5 inhibitors (e.g., atazanavir, clarithromycin, indinavir, itraconazole, nefazodone, nelfinavir, ritonavir, saquinavir, and telithromycin). The dosage of ONGLYZA should be limited to 2.5 mg when coadministered with a strong CYP3A4/5 inhibitor [see Dosage and Administration (2.3) and Clinical Pharmacology (12.3)].
7.2 Insulin or Insulin Secretagogues
Insulin and insulin secretagogues are known to cause hypoglycemia. Concomitant use of ONGLYZA with insulin or an insulin secretagogue may require lower dosages of insulin or the insulin secretagogue to reduce the risk of hypoglycemia [see Warnings and Precautions (5.3)].
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
Limited data with ONGLYZA in pregnant women are not sufficient to determine a drug-associated risk for major birth defects or miscarriages. There are risks to the mother and fetus associated with poorly controlled diabetes in pregnancy [see Clinical Considerations].
No adverse developmental effects independent of maternal toxicity were observed when saxagliptin was administered to pregnant rats and rabbits during the period of organogenesis and in pregnant and lactating rats during the pre- and postnatal period [see Data].
The estimated background risk of major birth defects is 6 to 10% in women with pre-gestational diabetes with an HbA1c greater than 7 and has been reported to be as high as 20 to 25% in women with an HbA1c greater than 10. The estimated background risk of miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
Poorly controlled diabetes in pregnancy increases the maternal risk for diabetic ketoacidosis, preeclampsia, spontaneous abortions, preterm delivery, still birth and delivery complications. Poorly controlled diabetes increases the fetal risk for major birth defects, stillbirth, and macrosomia related morbidity.
Data
Animal Data
In embryo-fetal development studies, saxagliptin was administered to pregnant rats and rabbits during the period of organogenesis, corresponding to the first trimester of human pregnancy. No adverse developmental effects were observed
in either species at exposures 1503- and 152-times the 5 mg clinical dose in rats and rabbits, respectively, based on AUC. Saxagliptin crosses the placenta into the fetus following dosing in pregnant rats.
In a prenatal and postnatal development study, no adverse developmental effects were observed in maternal rats administered saxagliptin from gestation day 6 through lactation day 21 at exposures up to 470-times the 5 mg clinical dose, based on AUC.
8.2 Lactation
Risk Summary
There is no information regarding the presence of ONGLYZA in human milk, the effects on the breastfed infant, or the effects on milk production.
Saxagliptin is present in the milk of lactating rats [see Data]. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for ONGLYZA and any potential adverse effects on the breastfed infant from ONGLYZA or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of ONGLYZA as an adjunct to diet and exercise to improve glycemic control in patients with type 2 diabetes mellitus have not been established in pediatric patients.
Effectiveness of ONGLYZA was not demonstrated in a 26-week, placebo-controlled, double-blind randomized clinical trial with a 26-week safety extension (NCT03199053) in 164 pediatric patients aged 10 to 17 years with inadequately controlled type 2 diabetes mellitus.
8.5 Geriatric Use
In the seven, double-blind, controlled clinical safety and efficacy trials of ONGLYZA, a total of 4751 (42.0%) of the 11301 patients randomized to ONGLYZA were 65 years and over, and 1210 (10.7%) were 75 years and over. No overall differences in safety or effectiveness were observed between patients 65 years of age and older and younger adult patients.
Saxagliptin and its active metabolite are eliminated in part by the kidney. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection in the elderly based on renal function [see Dosage and Administration (2.2) and Clinical Pharmacology (12.3)].
8.6 Renal Impairment
In a 12-week randomized placebo-controlled trial, ONGLYZA 2.5 mg was administered orally to 85 patients with moderate (n=48) or severe (n=18) renal impairment or end-stage renal disease (ESRD) (n=19) [see Clinical Studies (14)]. The incidence of adverse events, including serious adverse events and discontinuations due to adverse events, was similar between ONGLYZA and placebo. The overall incidence of reported hypoglycemia was 20% among patients treated with ONGLYZA 2.5 mg and 22% among patients treated with placebo. Four ONGLYZA-treated patients (4.7%) and three placebo-treated patients (3.5%) reported at least one episode of confirmed symptomatic hypoglycemia (accompanying fingerstick glucose ≤50 mg/dL).
10. Overdosage
In a controlled clinical trial, once-daily, orally-administered ONGLYZA in healthy patients at doses up to 400 mg daily for 2 weeks (80-times the MRHD) had no dose-related clinical adverse reactions and no clinically meaningful effect on QTc interval or heart rate.
In the event of an overdose, initiate appropriate supportive treatment as dictated by the patient’s clinical status. Saxagliptin and its active metabolite are removed by hemodialysis (23% of dose over 4 hours). Contact the Poison Help Line, (1-800-222-1222) or a medical toxicologist for additional overdosage management recommendations.
11. Onglyza Description
Saxagliptin is an orally-active inhibitor of the DPP-4 enzyme.
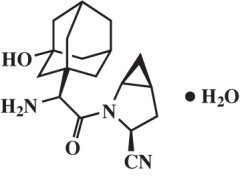
Saxagliptin monohydrate is described chemically as (1S,3S,5S)-2-[(2S)-2-Amino-2-(3-hydroxytricyclo[3.3.1.13,7]dec-1-yl)acetyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile, monohydrate or (1S,3S,5S)-2-[(2S)-2-Amino-2-(3-hydroxyadamantan-1-yl)acetyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile hydrate. The empirical formula is C18H25N3O2•H2O and the molecular weight is 333.43. The structural formula is:
Saxagliptin monohydrate is a white to light yellow or light brown, non-hygroscopic, crystalline powder. It is sparingly soluble in water at 24°C ± 3°C, slightly soluble in ethyl acetate, and soluble in methanol, ethanol, isopropyl alcohol, acetonitrile, acetone, and polyethylene glycol 400 (PEG 400).
Each film-coated tablet of ONGLYZA for oral use contains either 2.79 mg saxagliptin hydrochloride (anhydrous) equivalent to 2.5 mg saxagliptin or 5.58 mg saxagliptin hydrochloride (anhydrous) equivalent to 5 mg saxagliptin and the following inactive ingredients: croscarmellose sodium, lactose monohydrate, magnesium stearate and microcrystalline cellulose. In addition, the film coating contains the following inactive ingredients: iron oxides, polyethylene glycol, polyvinyl alcohol, talc, and titanium dioxide.
12. Onglyza - Clinical Pharmacology
12.1 Mechanism of Action
Increased concentrations of the incretin hormones such as glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) are released into the bloodstream from the small intestine in response to meals. These hormones cause insulin release from the pancreatic beta cells in a glucose-dependent manner but are inactivated by the DPP-4 enzyme within minutes. GLP-1 also lowers glucagon secretion from pancreatic alpha cells, reducing hepatic glucose production. In patients with type 2 diabetes mellitus, concentrations of GLP-1 are reduced but the insulin response to GLP-1 is preserved. Saxagliptin is a competitive DPP-4 inhibitor that slows the inactivation of the incretin hormones, thereby increasing their bloodstream concentrations and reducing fasting and postprandial glucose concentrations in a glucose-dependent manner in patients with type 2 diabetes mellitus.
12.2 Pharmacodynamics
In patients with type 2 diabetes mellitus, administration of ONGLYZA inhibits DPP-4 enzyme activity for a 24-hour period. After an oral glucose load or a meal, this DPP-4 inhibition resulted in a 2- to 3-fold increase in circulating levels of active GLP-1 and GIP, decreased glucagon concentrations, and increased glucose-dependent insulin secretion from pancreatic beta cells. The rise in insulin and decrease in glucagon were associated with lower fasting glucose concentrations and reduced glucose excursion following an oral glucose load or a meal.
Cardiac Electrophysiology
In a randomized, double-blind, placebo-controlled, 4-way crossover, active comparator trial using moxifloxacin in 40 healthy subjects, ONGLYZA was not associated with clinically meaningful prolongation of the QTc interval or heart rate at daily doses up to 40 mg (8-times the MRHD).
12.3 Pharmacokinetics
The pharmacokinetics of saxagliptin and its active metabolite, 5-hydroxy saxagliptin were similar in healthy subjects and in patients with type 2 diabetes mellitus. The Cmax and AUC values of saxagliptin and its active metabolite increased proportionally in the 2.5 to 400 mg dose range. Following a 5 mg single oral dose of saxagliptin to healthy subjects, the mean plasma AUC values for saxagliptin and its active metabolite were 78 ng•h/mL and 214 ng•h/mL, respectively. The corresponding plasma Cmax values were 24 ng/mL and 47 ng/mL, respectively. The average variability (%CV) for AUC and Cmax for both saxagliptin and its active metabolite was less than 25%.
No appreciable accumulation of either saxagliptin or its active metabolite was observed with repeated once-daily dosing at any dose level. No dose- and time-dependence were observed in the clearance of saxagliptin and its active metabolite over 14 days of once-daily dosing with saxagliptin at doses ranging from 2.5 to 400 mg.
Absorption
The median time to maximum concentration (Tmax) following the 5 mg once daily dose was 2 hours for saxagliptin and 4 hours for its active metabolite.
Effect of Food
Administration with a high-fat meal resulted in an increase in Tmax of saxagliptin by approximately 20 minutes as compared to fasted conditions. There was a 27% increase in the AUC of saxagliptin when given with a meal as compared to fasted conditions. ONGLYZA may be administered with or without food.
Distribution
The in vitro protein binding of saxagliptin and its active metabolite in human serum is negligible. Therefore, changes in blood protein levels in various disease states (e.g., renal or hepatic impairment) are not expected to alter the disposition of saxagliptin.
Metabolism
The metabolism of saxagliptin is primarily mediated by cytochrome P450 3A4/5 (CYP3A4/5). The major metabolite of saxagliptin is also a DPP-4 inhibitor, which is one-half as potent as saxagliptin. Therefore, strong CYP3A4/5 inhibitors and inducers will alter the pharmacokinetics of saxagliptin and its active metabolite [see Drug Interactions (7.1)].
Excretion
Saxagliptin is eliminated by both renal and hepatic pathways. Following a single 50 mg dose of 14C-saxagliptin, 24%, 36%, and 75% of the dose was excreted in the urine as saxagliptin, its active metabolite, and total radioactivity, respectively. The average renal clearance of saxagliptin (~230 mL/min) was greater than the average estimated glomerular filtration rate (~120 mL/min), suggesting some active renal excretion. A total of 22% of the administered radioactivity was recovered in feces representing the fraction of the saxagliptin dose excreted in bile and/or unabsorbed drug from the gastrointestinal tract. Following a single oral dose of ONGLYZA 5 mg to healthy subjects, the mean plasma terminal half-life (t1/2) for saxagliptin and its active metabolite was 2.5 and 3.1 hours, respectively.
Specific Populations
Geriatric Patients
No dosage adjustment is recommended based on age alone. Elderly subjects (65-80 years) had 23% and 59% higher geometric mean Cmax and geometric mean AUC values, respectively, for saxagliptin than young subjects (18-40 years). Differences in active metabolite pharmacokinetics between elderly and young subjects generally reflected the differences observed in saxagliptin pharmacokinetics. The difference between the pharmacokinetics of saxagliptin and the active metabolite in young and elderly subjects is likely due to multiple factors including declining renal function and metabolic capacity with increasing age. Age was not identified as a significant covariate on the apparent clearance of saxagliptin and its active metabolite in the population pharmacokinetic analysis.
Male and Female Patients
No dosage adjustment is recommended based on gender. There were no differences observed in saxagliptin pharmacokinetics between males and females. Compared to males, females had approximately 25% higher exposure values for the active metabolite than males, but this difference is unlikely to be of clinical relevance. Gender was not identified as a significant covariate on the apparent clearance of saxagliptin and its active metabolite in the population pharmacokinetic analysis.
Racial or Ethnic Groups
No dosage adjustment is recommended based on race. The population pharmacokinetic analysis compared the pharmacokinetics of saxagliptin and its active metabolite in 309 White subjects with 105 subjects of other races (consisting of six racial groups). No significant difference in the pharmacokinetics of saxagliptin and its active metabolite were detected between these two populations.
Patients with Renal Impairment
A single-dose, open-label study was conducted to evaluate the pharmacokinetics of saxagliptin (10 mg dose) in subjects with varying degrees of chronic renal impairment compared to subjects with normal renal function. The 10 mg dosage is not an approved dosage.
The degree of renal impairment did not affect Cmax of saxagliptin or its metabolite. In subjects with moderate renal impairment with (eGFR 30 to less than 45 mL/min/1.73 m2), severe renal impairment (eGFR 15 to less than 30 mL/min/1.73 m2) and ESRD patient on hemodialysis, the AUC values of saxagliptin or its active metabolite were >2 fold higher than AUC values in subjects with normal renal function.
Patients with Hepatic Impairment
In subjects with hepatic impairment (Child-Pugh classes A, B, and C), mean Cmax and AUC of saxagliptin were up to 8% and 77% higher, respectively, compared to healthy matched controls following administration of a single 10 mg dose of saxagliptin. The 10 mg dosage is not an approved dosage. The corresponding Cmax and AUC of the active metabolite were up to 59% and 33% lower, respectively, compared to healthy matched controls. These differences are not considered to be clinically meaningful.
Drug Interaction Studies
In Vitro Assessment of Drug Interactions
The metabolism of saxagliptin is primarily mediated by CYP3A4/5.
In in vitro studies, saxagliptin and its active metabolite did not inhibit CYP1A2, 2A6, 2B6, 2C9, 2C19, 2D6, 2E1, or 3A4, or induce CYP1A2, 2B6, 2C9, or 3A4. Therefore, saxagliptin is not expected to alter the metabolic clearance of coadministered drugs that are metabolized by these enzymes. Saxagliptin is a P-glycoprotein (P-gp) substrate but is not a significant inhibitor or inducer of P-gp.
In Vivo Assessment of Drug Interactions
| Coadministered Drug | Dosage of
Coadministered Drug* | Dosage of
Saxagliptin* | Geometric Mean Ratio
(ratio with/without coadministered drug) No Effect = 1.00 |
||
|---|---|---|---|---|---|
| AUC† | Cmax | ||||
|
|||||
|
No dosing adjustments required for the following: |
|||||
|
Metformin |
1,000 mg |
100 mg |
saxagliptin |
0.98 |
0.79 |
|
Glyburide |
5 mg |
10 mg |
saxagliptin |
0.98 |
1.08 |
|
Dapagliflozin |
10 mg single dose |
5 mg single dose |
saxagliptin 5-hydroxy saxagliptin |
↓1% ↑9% |
↓7% ↑6% |
|
Pioglitazone‡ |
45 mg QD for 10 days |
10 mg QD for 5 days |
saxagliptin |
1.11 |
1.11 |
|
Digoxin |
0.25 mg q6h first day followed by q12h second day followed by QD for |
10 mg QD for 7 days |
saxagliptin |
1.05 |
0.99 |
|
Simvastatin |
40 mg QD for 8 days |
10 mg QD for 4 days |
saxagliptin |
1.12 |
1.21 |
|
Diltiazem |
360 mg LA QD for 9 days |
10 mg |
saxagliptin |
2.09 |
1.63 |
|
Rifampin§ |
600 mg QD for 6 days |
5 mg |
saxagliptin |
0.24 |
0.47 |
|
Omeprazole |
40 mg QD for 5 days |
10 mg |
saxagliptin |
1.13 |
0.98 |
|
Aluminum hydroxide + magnesium hydroxide + simethicone |
aluminum hydroxide: |
10 mg |
saxagliptin |
0.97 |
0.74 |
|
Famotidine |
40 mg |
10 mg |
saxagliptin |
1.03 |
1.14 |
|
Limit ONGLYZA dose to 2.5 mg once daily when coadministered with strong CYP3A4/5 inhibitors [see Drug Interactions (7.1) and Dosage and Administration (2.3)]: |
|||||
|
Ketoconazole |
200 mg BID for 9 days |
100 mg |
saxagliptin |
2.45 |
1.62 |
|
Ketoconazole |
200 mg BID for 7 days |
20 mg |
saxagliptin |
3.67 |
2.44 |
ND=not determined; QD=once daily; q6h=every 6 hours; q12h=every 12 hours; BID=twice daily; LA=long acting
| Coadministered Drug | Dosage of Coadministered Drug* | Dosage of Saxagliptin* | Geometric Mean Ratio (ratio with/without saxagliptin) No Effect = 1.00 |
||
|---|---|---|---|---|---|
| AUC† | Cmax | ||||
|
No dosing adjustments required for the following: |
|||||
|
Metformin |
1,000 mg |
100 mg |
metformin |
1.20 |
1.09 |
|
Glyburide |
5 mg |
10 mg |
glyburide |
1.06 |
1.16 |
|
Pioglitazone‡ |
45 mg QD for 10 days |
10 mg QD |
pioglitazone |
1.08 |
1.14 |
|
Digoxin |
0.25 mg q6h first day followed by q12h second day followed by QD for 5 days |
10 mg QD |
digoxin |
1.06 |
1.09 |
|
Simvastatin |
40 mg QD for 8 days |
10 mg QD |
simvastatin |
1.04 |
0.88 |
|
Diltiazem |
360 mg LA QD for 9 days |
10 mg |
diltiazem |
1.10 |
1.16 |
|
Ketoconazole |
200 mg BID for 9 days |
100 mg |
ketoconazole |
0.87 |
0.84 |
|
Ethinyl estradiol and Norgestimate |
ethinyl estradiol 0.035 mg and norgestimate 0.250 mg |
5 mg QD for 21 days |
ethinyl estradiol |
1.07 |
0.98 |
ND=not determined; QD=once daily; q6h=every 6 hours; q12h=every 12 hours; BID=twice daily; LA=long acting
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Carcinogenicity was evaluated in 2-year studies conducted in CD-1 mice and Sprague-Dawley rats. Saxagliptin did not increase the incidence of tumors in mice dosed orally at 50, 250, and 600 mg/kg up to 870-times (males) and 1165-times (females) the 5 mg/day clinical dose, based on AUC. Saxagliptin did not increase the incidence of tumors in rats dosed orally at 25, 75, 150, and 300 mg/kg up to 355-times (males) and 2217-times (females) the 5 mg/day clinical dose, based on AUC.
Mutagenesis
Saxagliptin was not mutagenic or clastogenic in a battery of genotoxicity tests (Ames bacterial mutagenesis, human and rat lymphocyte cytogenetics, rat bone marrow micronucleus and DNA repair assays). The active metabolite of saxagliptin was not mutagenic in an Ames bacterial assay.
Impairment of Fertility
Saxagliptin administered to rats had no effect on fertility or the ability to maintain a litter at exposures up to 603-times and 776-times the 5 mg clinical dose in males and females, based on AUC.
13.2 Animal Toxicology and/or Pharmacology
Saxagliptin produced adverse skin changes in the extremities of cynomolgus monkeys (scabs and/or ulceration of tail, digits, scrotum, and/or nose). Skin lesions were reversible within exposure approximately 20-times the 5 mg clinical dose, but in some cases were irreversible and necrotizing at higher exposures. Adverse skin changes were not observed at exposures similar to (1- to 3-times) the 5 mg clinical dose. Clinical correlates to skin lesions in monkeys have not been observed in human clinical trials of saxagliptin.
14. Clinical Studies
14.1 Glycemic Control Trials in Adults with Type 2 Diabetes Mellitus
ONGLYZA has been studied as monotherapy and in combination with metformin HCl, glyburide, and thiazolidinedione (pioglitazone and rosiglitazone) therapy.
A total of 4148 patients with type 2 diabetes mellitus were randomized in six, double-blind, controlled clinical trials conducted to evaluate the safety and glycemic efficacy of ONGLYZA administered orally. A total of 3021 patients in these trials were treated with ONGLYZA. In these trials, the mean age was 54 years, and 71% of patients were White, 16% were Asian, 4% were Black or African American, and 9% were of other racial groups. An additional 423 patients, including 315 who received ONGLYZA, participated in a placebo-controlled, dose-ranging trial of 6 to 12 weeks in duration.
In these six, double-blind trials, ONGLYZA was evaluated at doses of 2.5 mg and 5 mg once daily. Three of these trials also evaluated an ONGLYZA dose of 10 mg daily. The 10 mg daily dose of ONGLYZA did not provide greater efficacy than the 5 mg daily dose. The 10 mg dosage is not an approved dosage. Treatment with ONGLYZA 5 mg and 2.5 mg doses produced statistically significant improvements in A1C, fasting plasma glucose (FPG), and 2-hour postprandial glucose (PPG) following a standard oral glucose tolerance test (OGTT), compared to control. Reductions in A1C were seen across subgroups including gender, age, race, and baseline BMI.
ONGLYZA was not associated with significant changes from baseline in body weight or fasting serum lipids compared to placebo.
ONGLYZA has also been evaluated in five additional trials in patients with type 2 diabetes mellitus: an active-controlled trial comparing add-on therapy with ONGLYZA to glipizide in 858 patients inadequately controlled on metformin HCl alone, a trial comparing ONGLYZA to placebo in 455 patients inadequately controlled on insulin alone or on insulin in combination with metformin HCl, a trial comparing ONGLYZA to placebo in 257 patients inadequately controlled on metformin HCl plus a sulfonylurea, a trial comparing ONGLYZA to placebo in 315 patients inadequately controlled on dapagliflozin and metformin HCl, and a trial comparing ONGLYZA to placebo in 170 patients with type 2 diabetes mellitus and moderate or severe renal impairment or ESRD.
Monotherapy
A total of 766 patients with type 2 diabetes mellitus inadequately controlled on diet and exercise (A1C ≥7% to ≤10%) participated in two 24-week, double-blind, placebo-controlled trials evaluating the efficacy and safety of ONGLYZA monotherapy.
In the first trial, following a 2-week single-blind diet, exercise, and placebo lead-in period, 401 patients were randomized to 2.5 mg, 5 mg, or 10 mg of ONGLYZA or placebo. The 10 mg dosage is not an approved dosage. Patients who failed to meet specific glycemic goals during the trial were treated with metformin HCl rescue therapy, added on to placebo or ONGLYZA. Efficacy was evaluated at the last measurement prior to rescue therapy for patients needing rescue. Dose titration of ONGLYZA was not permitted.
Treatment with ONGLYZA 2.5 mg and 5 mg daily provided significant improvements in A1C, FPG, and PPG compared to placebo (Table 4). The percentage of patients who discontinued for lack of glycemic control or who were rescued for meeting prespecified glycemic criteria was 16% in the ONGLYZA 2.5 mg treatment group, 20% in the ONGLYZA 5 mg treatment group, and 26% in the placebo group.
|
|||
|
Efficacy Parameter |
ONGLYZA
|
ONGLYZA
|
Placebo
|
|
Hemoglobin A1C (%) |
N=100 |
N=103 |
N=92 |
|
Baseline (mean) |
7.9 |
8.0 |
7.9 |
|
Change from baseline (adjusted mean†) |
−0.4 |
−0.5 |
+0.2 |
|
Difference from placebo (adjusted mean†) |
−0.6‡ |
−0.6‡ | |
|
95% Confidence Interval |
(−0.9, −0.3) |
(−0.9, −0.4) | |
|
Percent of patients achieving A1C <7% |
35% (35/100) |
38%§ (39/103) |
24% (22/92) |
|
Fasting Plasma Glucose (mg/dL) |
N=101 |
N=105 |
N=92 |
|
Baseline (mean) |
178 |
171 |
172 |
|
Change from baseline (adjusted mean†) |
−15 |
−9 |
+6 |
|
Difference from placebo (adjusted mean†) |
−21§ |
−15§ | |
|
95% Confidence Interval |
(−31, −10) |
(−25, −4) | |
|
2-hour Postprandial Glucose (mg/dL) |
N=78 |
N=84 |
N=71 |
|
Baseline (mean) |
279 |
278 |
283 |
|
Change from baseline (adjusted mean†) |
−45 |
−43 |
−6 |
|
Difference from placebo (adjusted mean†) |
−39¶ |
−37§ | |
|
95% Confidence Interval |
(−61, −16) |
(−59, −15) | |
A second 24-week monotherapy trial was conducted to assess a range of dosing regimens for ONGLYZA.
Treatment-naive patients with inadequately controlled type 2 diabetes mellitus (A1C ≥7% to ≤10%) underwent a 2-week, single-blind diet, exercise, and placebo lead-in period. A total of 365 patients were randomized to 2.5 mg every morning, 5 mg every morning, 2.5 mg with possible titration to 5 mg every morning, or 5 mg every evening of ONGLYZA, or placebo. Patients who failed to meet specific glycemic goals during the trial were treated with metformin HCl rescue therapy added on to placebo or ONGLYZA; the number of patients randomized per treatment group ranged from 71 to 74.
Treatment with either ONGLYZA 5 mg every morning or 5 mg every evening provided significant improvements in A1C versus placebo (mean placebo-corrected reductions of −0.4% and −0.3%, respectively). Treatment with ONGLYZA 2.5 mg every morning also provided significant improvement in A1C versus placebo (mean placebo-corrected reduction of −0.4%).
Combination Therapy
Add-On Combination Therapy with Metformin HCl
A total of 743 patients with type 2 diabetes mellitus participated in this 24-week, randomized, double-blind, placebo-controlled trial to evaluate the efficacy and safety of ONGLYZA in combination with metformin HCl in patients with inadequate glycemic control (A1C ≥7% and ≤10%) on metformin alone. To qualify for enrollment, patients were required to be on a stable dose of metformin HCl (1,500-2,550 mg daily) for at least 8 weeks.
Patients who met eligibility criteria were enrolled in a single-blind, 2-week, dietary and exercise placebo lead-in period during which patients received metformin HCl at their pre-trial dose, up to 2,500 mg daily. Following the lead-in period, eligible patients were randomized to 2.5 mg, 5 mg, or 10 mg of ONGLYZA or placebo in addition to their current dose of open-label metformin HCl. The 10 mg dosage of ONGLYZA is not an approved dosage. Patients who failed to meet specific glycemic goals during the trial were treated with pioglitazone rescue therapy, added on to existing trial medications. Dose titrations of ONGLYZA and metformin HCl were not permitted.
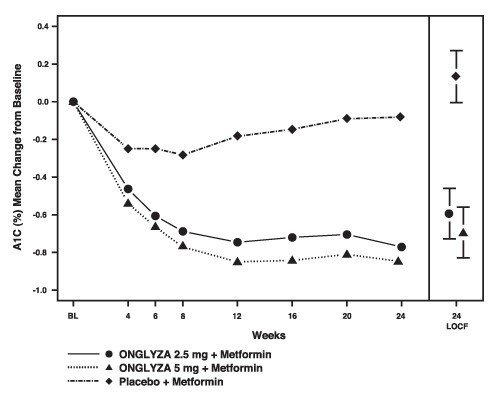
ONGLYZA 2.5 mg and 5 mg add-on to metformin provided significant improvements in A1C, FPG, and PPG compared with placebo add-on to metformin (Table 5). Mean changes from baseline for A1C over time and at endpoint are shown in Figure 1. The proportion of patients who discontinued for lack of glycemic control or who were rescued for meeting prespecified glycemic criteria was 15% in the ONGLYZA 2.5 mg add-on to metformin group, 13% in the ONGLYZA 5 mg add-on to metformin group, and 27% in the placebo add-on to metformin group.
| Efficacy Parameter | ONGLYZA 2.5 mg
+ Metformin HCl N=192 | ONGLYZA 5 mg
+ Metformin HCl N=191 | Placebo
+ Metformin HCl N=179 |
|---|---|---|---|
|
|||
|
Hemoglobin A1C (%) |
N=186 |
N=186 |
N=175 |
|
Baseline (mean) |
8.1 |
8.1 |
8.1 |
|
Change from baseline (adjusted mean†) |
−0.6 |
−0.7 |
+0.1 |
|
Difference from placebo (adjusted mean†) |
−0.7‡ |
−0.8‡ | |
|
95% Confidence Interval |
(−0.9, −0.5) |
(−1.0, −0.6) | |
|
Percent of patients achieving A1C <7% |
37%§ (69/186) |
44%§ (81/186) |
17% (29/175) |
|
Fasting Plasma Glucose (mg/dL) |
N=188 |
N=187 |
N=176 |
|
Baseline (mean) |
174 |
179 |
175 |
|
Change from baseline (adjusted mean†) |
−14 |
−22 |
+1 |
|
Difference from placebo (adjusted mean†) |
−16§ |
−23§ | |
|
95% Confidence Interval |
(−23, −9) |
(−30, −16) | |
|
2-hour Postprandial Glucose (mg/dL) |
N=155 |
N=155 |
N=135 |
|
Baseline (mean) |
294 |
296 |
295 |
|
Change from baseline (adjusted mean†) |
−62 |
−58 |
−18 |
|
Difference from placebo (adjusted mean†) |
−44§ |
−40§ | |
|
95% Confidence Interval |
(−60, −27) |
(−56, −24) | |
Figure 1: Mean Change from Baseline in A1C in a Placebo-Controlled Trial of ONGLYZA as Add-On Combination Therapy with Metformin HCl*
*Includes patients with a baseline and week 24 value.
Week 24 (LOCF) includes intent-to-treat population using last observation on trial prior to pioglitazone rescue therapy for patients needing rescue. Mean change from baseline is adjusted for baseline value.
Add-On Combination Therapy with a Thiazolidinedione
A total of 565 patients with type 2 diabetes mellitus participated in this 24-week, randomized, double-blind, placebo-controlled trial to evaluate the efficacy and safety of ONGLYZA in combination with a thiazolidinedione (TZD) in patients with inadequate glycemic control (A1C ≥7% to ≤10.5%) on TZD alone. To qualify for enrollment, patients were required to be on a stable dose of pioglitazone (30-45 mg once daily) or rosiglitazone (4 mg once daily or 8 mg either once daily or in two divided doses of 4 mg) for at least 12 weeks.
Patients who met eligibility criteria were enrolled in a single-blind, 2-week, dietary and exercise placebo lead-in period during which patients received TZD at their pre-trial dose. Following the lead-in period, eligible patients were randomized to 2.5 mg or 5 mg of ONGLYZA or placebo in addition to their current dose of TZD. Patients who failed to meet specific glycemic goals during the trial were treated with metformin HCl rescue, added on to existing trial medications. Dose titration of ONGLYZA or TZD was not permitted during the trial. A change in TZD regimen from rosiglitazone to pioglitazone at specified, equivalent therapeutic doses was permitted at the investigator’s discretion if believed to be medically appropriate.
ONGLYZA 2.5 mg and 5 mg add-on to TZD provided significant improvements in A1C, FPG, and PPG compared with placebo add-on to TZD (Table 6). The proportion of patients who discontinued for lack of glycemic control or who were rescued for meeting prespecified glycemic criteria was 10% in the ONGLYZA 2.5 mg add-on to TZD group, 6% for the ONGLYZA 5 mg add-on to TZD group, and 10% in the placebo add-on to TZD group.
| Efficacy Parameter | ONGLYZA 2.5 mg
+ TZD N=195 | ONGLYZA 5 mg
+ TZD N=186 | Placebo
+ TZD N=184 |
|---|---|---|---|
|
Hemoglobin A1C (%) |
N=192 |
N=183 |
N=180 |
|
Baseline (mean) |
8.3 |
8.4 |
8.2 |
|
Change from baseline (adjusted mean†) |
−0.7 |
−0.9 |
−0.3 |
|
Difference from placebo (adjusted mean†) |
−0.4‡ |
−0.6§ | |
|
95% Confidence Interval |
(−0.6, −0.2) |
(−0.8, −0.4) | |
|
Percent of patients achieving A1C <7% |
42%‡ (81/192) |
42% (77/184) |
26% (46/180) |
|
Fasting Plasma Glucose (mg/dL) |
N=193 |
N=185 |
N=181 |
|
Baseline (mean) |
163 |
160 |
162 |
|
Change from baseline (adjusted mean†) |
−14 |
−17 |
−3 |
|
Difference from placebo (adjusted mean†) |
−12‡ |
−15‡ | |
|
95% Confidence Interval |
(−20, −3) |
(−23, −6) | |
|
2-hour Postprandial Glucose (mg/dL) |
N=156 |
N=134 |
N=127 |
|
Baseline (mean) |
296 |
303 |
291 |
|
Change from baseline (adjusted mean†) |
−55 |
−65 |
−15 |
|
Difference from placebo (adjusted mean†) |
−40‡ |
−50‡ | |
|
95% Confidence Interval |
(−56, −24) |
(−66, −34) | |
Add-On Combination Therapy with Glyburide
A total of 768 patients with type 2 diabetes mellitus participated in this 24-week, randomized, double-blind, placebo-controlled trial to evaluate the efficacy and safety of ONGLYZA in combination with a sulfonylurea (SU) in patients with inadequate glycemic control at enrollment (A1C ≥7.5% to ≤10%) on a submaximal dose of SU alone. To qualify for enrollment, patients were required to be on a submaximal dose of SU for 2 months or greater. In this trial, ONGLYZA in combination with a fixed, intermediate dose of SU was compared to titration to a higher dose of SU.
Patients who met eligibility criteria were enrolled in a single-blind, 4-week, dietary and exercise lead-in period, and placed on glyburide 7.5 mg once daily. Following the lead-in period, eligible patients with A1C ≥7% to ≤10% were randomized to either 2.5 mg or 5 mg of ONGLYZA add-on to 7.5 mg glyburide or to placebo plus a 10 mg total daily dose of glyburide. Patients who received placebo were eligible to have glyburide up-titrated to a total daily dose of 15 mg. Up-titration of glyburide was not permitted in patients who received ONGLYZA 2.5 mg or 5 mg. Glyburide could be down-titrated in any treatment group once during the 24-week trial period due to hypoglycemia as deemed necessary by the investigator. Approximately 92% of patients in the placebo plus glyburide group were up-titrated to a final total daily dose of 15 mg during the first 4 weeks of the trial period. Patients who failed to meet specific glycemic goals during the trial were treated with metformin HCl rescue, added on to existing trial medication. Dose titration of ONGLYZA was not permitted during the trial.
In combination with glyburide, ONGLYZA 2.5 mg and 5 mg provided significant improvements in A1C, FPG, and PPG compared with the placebo plus up-titrated glyburide group (Table 7). The proportion of patients who discontinued for lack of glycemic control or who were rescued for meeting prespecified glycemic criteria was 18% in the ONGLYZA 2.5 mg add-on to glyburide group, 17% in the ONGLYZA 5 mg add-on to glyburide group, and 30% in the placebo plus up-titrated glyburide group.
| Efficacy Parameter | ONGLYZA
2.5 mg + Glyburide 7.5 mg N=248 | ONGLYZA
5 mg + Glyburide 7.5 mg N=253 | Placebo
+ Up-Titrated Glyburide N=267 |
|---|---|---|---|
|
|||
|
Hemoglobin A1C (%) |
N=246 |
N=250 |
N=264 |
|
Baseline (mean) |
8.4 |
8.5 |
8.4 |
|
Change from baseline (adjusted mean†) |
−0.5 |
−0.6 |
+0.1 |
|
Difference from up-titrated glyburide (adjusted mean†) |
−0.6‡ |
−0.7‡ | |
|
95% Confidence Interval |
(−0.8, −0.5) |
(−0.9, −0.6) | |
|
Percent of patients achieving A1C <7% |
22%§ (55/246) |
23%§ (57/250) |
9% (24/264) |
|
Fasting Plasma Glucose (mg/dL) |
N=247 |
N=252 |
N=265 |
|
Baseline (mean) |
170 |
175 |
174 |
|
Change from baseline (adjusted mean†) |
−7 |
−10 |
+1 |
|
Difference from up-titrated glyburide (adjusted mean†) |
−8§ |
−10§ | |
|
95% Confidence Interval |
(−14, −1) |
(−17, −4) | |
|
2-hour Postprandial Glucose (mg/dL) |
N=195 |
N=202 |
N=206 |
|
Baseline (mean) |
309 |
315 |
323 |
|
Change from baseline (adjusted mean†) |
−31 |
−34 |
+8 |
|
Difference from up-titrated glyburide (adjusted mean†) |
−38§ |
−42§ | |
|
95% Confidence Interval |
(−50, −27) |
(−53, −31) | |
Coadministration with Metformin HCl in Treatment-Naive Patients
A total of 1306 treatment-naive patients with type 2 diabetes mellitus participated in this 24-week, randomized, double-blind, active-controlled trial to evaluate the efficacy and safety of ONGLYZA coadministered with metformin HCl in patients with inadequate glycemic control (A1C ≥8% to ≤12%) on diet and exercise alone. Patients were required to be treatment-naive to be enrolled in this trial.
Patients who met eligibility criteria were enrolled in a single-blind, 1-week, dietary and exercise placebo lead-in period. Patients were randomized to one of four treatment arms: ONGLYZA 5 mg + metformin HCl 500 mg, saxagliptin 10 mg + metformin HCl 500 mg, saxagliptin 10 mg + placebo, or metformin HCl 500 mg + placebo. The 10 mg saxagliptin dosage is not an approved dosage. ONGLYZA was dosed once daily. In the 3 treatment groups using metformin HCl, the metformin HCl dose was up-titrated weekly in 500 mg per day increments, as tolerated, to a maximum of 2,000 mg per day based on FPG. Patients who failed to meet specific glycemic goals during the studies were treated with pioglitazone rescue as add-on therapy.
Coadministration of ONGLYZA 5 mg plus metformin HCl provided significant improvements in A1C, FPG, and PPG compared with placebo plus metformin (Table 8).
| Efficacy Parameter | ONGLYZA 5 mg
+ Metformin HCl N=320 | Placebo
+ Metformin HCl N=328 |
|---|---|---|
|
||
|
Hemoglobin A1C (%) |
N=306 |
N=313 |
|
Baseline (mean) |
9.4 |
9.4 |
|
Change from baseline (adjusted mean†) |
−2.5 |
−2.0 |
|
Difference from placebo + metformin HCl (adjusted mean†) |
−0.5‡ | |
|
95% Confidence Interval |
(−0.7, −0.4) | |
|
Percent of patients achieving A1C <7% |
60%§ (185/307) |
41% (129/314) |
|
Fasting Plasma Glucose (mg/dL) |
N=315 |
N=320 |
|
Baseline (mean) |
199 |
199 |
|
Change from baseline (adjusted mean†) |
−60 |
−47 |
|
Difference from placebo + metformin HCl (adjusted mean†) |
−13§ | |
|
95% Confidence Interval |
(−19, −6) | |
|
2-hour Postprandial Glucose (mg/dL) |
N=146 |
N=141 |
|
Baseline (mean) |
340 |
355 |
|
Change from baseline (adjusted mean†) |
−138 |
−97 |
|
Difference from placebo + metformin HCl (adjusted mean†) |
−41§ | |
|
95% Confidence Interval |
(−57, −25) | |
Add-On Combination Therapy with Metformin HCl versus Glipizide Add-On Combination Therapy with Metformin HCl
In this 52-week, active-controlled trial, a total of 858 patients with type 2 diabetes mellitus and inadequate glycemic control (A1C >6.5% and ≤10%) on metformin HCl alone were randomized to double-blind add-on therapy with ONGLYZA or glipizide. Patients were required to be on a stable dose of metformin HCl (at least 1,500 mg daily) for at least 8 weeks prior to enrollment.
Patients who met eligibility criteria were enrolled in a single-blind, 2-week, dietary and exercise placebo lead-in period during which patients received metformin HCl (1,500-3,000 mg based on their pre-trial dose). Following the lead-in period, eligible patients were randomized to 5 mg of ONGLYZA or 5 mg of glipizide in addition to their current dose of open-label metformin HCl. Patients in the glipizide plus metformin HCl group underwent blinded titration of the glipizide dose during the first 18 weeks of the trial up to a maximum glipizide dose of 20 mg per day. Titration was based on a goal FPG ≤110 mg/dL or the highest tolerable glipizide dose. Fifty percent (50%) of the glipizide-treated patients were titrated to the 20-mg daily dose; 21% of the glipizide-treated patients had a final daily glipizide dose of 5 mg or less. The mean final daily dose of glipizide was 15 mg.
After 52 weeks of treatment, ONGLYZA and glipizide resulted in similar mean reductions from baseline in A1C when added to metformin HCl therapy (Table 9). This conclusion may be limited to patients with baseline A1C comparable to those in the trial (91% of patients had baseline A1C <9%).
From a baseline mean body weight of 89 kg, there was a statistically significant mean reduction of 1.1 kg in patients treated with ONGLYZA compared to a mean weight gain of 1.1 kg in patients treated with glipizide (p<0.0001).
| Efficacy Parameter | ONGLYZA 5 mg
+ Metformin HCl N=428 | Titrated Glipizide
+ Metformin HCl N=430 |
|---|---|---|
|
||
|
Hemoglobin A1C (%) |
N=423 |
N=423 |
|
Baseline (mean) |
7.7 |
7.6 |
|
Change from baseline (adjusted mean†) |
−0.6 |
−0.7 |
|
Difference from glipizide + metformin HCl (adjusted mean†) |
0.1 | |
|
95% Confidence Interval |
(−0.02, 0.2)‡ | |
|
Fasting Plasma Glucose (mg/dL) |
N=420 |
N=420 |
|
Baseline (mean) |
162 |
161 |
|
Change from baseline (adjusted mean†) |
−9 |
−16 |
|
Difference from glipizide + metformin HCl (adjusted mean†) |
6 | |
|
95% Confidence Interval |
(2, 11)§ | |
Add-On Combination Therapy with Insulin (with or without metformin HCl)
A total of 455 patients with type 2 diabetes mellitus participated in this 24-week, randomized, double-blind, placebo-controlled trial to evaluate the efficacy and safety of ONGLYZA in combination with insulin in patients with inadequate glycemic control (A1C ≥7.5% and ≤11%) on insulin alone (N=141) or on insulin in combination with a stable dose of metformin HCl (N=314). Patients were required to be on a stable dose of insulin (≥30 units to ≤150 units daily) with ≤20% variation in total daily dose for ≥8 weeks prior to screening. Patients entered the trial on intermediate- or long-acting (basal) insulin or premixed insulin. Patients using short-acting insulins were excluded unless the short-acting insulin was administered as part of a premixed insulin.
Patients who met eligibility criteria were enrolled in a single-blind, four-week, dietary and exercise placebo lead-in period during which patients received insulin (and metformin HCl if applicable) at their pretrial dose(s). Following the lead-in period, eligible patients were randomized to add-on therapy with either ONGLYZA 5 mg or placebo. Doses of the antidiabetic therapies were to remain stable but patients were rescued and allowed to adjust the insulin regimen if specific glycemic goals were not met or if the investigator learned that the patient had self-increased the insulin dose by >20%. Data after rescue were excluded from the primary efficacy analyses.
Add-on therapy with ONGLYZA 5 mg provided significant improvements from baseline to Week 24 in A1C and PPG compared with add-on placebo (Table 10). Similar mean reductions in A1C versus placebo were observed for patients using ONGLYZA 5 mg add-on to insulin alone and ONGLYZA 5 mg add-on to insulin in combination with metformin HCl (−0.4% and −0.4%, respectively). The percentage of patients who discontinued for lack of glycemic control or who were rescued was 23% in the ONGLYZA group and 32% in the placebo group.
The mean daily insulin dose at baseline was 53 units in patients treated with ONGLYZA 5 mg and 55 units in patients treated with placebo. The mean change from baseline in daily dose of insulin was 2 units for the ONGLYZA 5 mg group and 5 units for the placebo group.
|
||
|
Efficacy Parameter |
ONGLYZA 5 mg
|
Placebo
|
|
Hemoglobin A1C (%) |
N=300 |
N=149 |
|
Baseline (mean) |
8.7 |
8.7 |
|
Change from baseline (adjusted mean†) |
−0.7 |
−0.3 |
|
Difference from placebo (adjusted mean†) |
−0.4‡ | |
|
95% Confidence Interval |
(−0.6, −0.2) | |
|
2-hour Postprandial Glucose (mg/dL) |
N=262 |
N=129 |
|
Baseline (mean) |
251 |
255 |
|
Change from baseline (adjusted mean†) |
−27 |
−4 |
|
Difference from placebo (adjusted mean†) |
−23§ | |
|
95% Confidence Interval |
(−37, −9) | |
The change in fasting plasma glucose from baseline to Week 24 was also tested, but was not statistically significant. The percent of patients achieving an A1C <7% was 17% (52/300) with ONGLYZA in combination with insulin compared to 7% (10/149) with placebo. Significance was not tested.
Add-On Combination Therapy with Metformin HCl plus Sulfonylurea
A total of 257 patients with type 2 diabetes mellitus participated in this 24-week, randomized, double-blind, placebo-controlled trial to evaluate the efficacy and safety of ONGLYZA in combination with metformin HCl plus a sulfonylurea in patients with inadequate glycemic control (A1C ≥7% and ≤10%). Patients were to be on a stable combined dose of metformin HCl extended-release or immediate-release (at maximum tolerated dose, with minimum dose for enrollment being 1,500 mg) and a sulfonylurea (at maximum tolerated dose, with minimum dose for enrollment being ≥50% of the maximum recommended dose) for ≥8 weeks prior to enrollment.
Patients who met eligibility criteria were entered in a 2-week enrollment period to allow assessment of inclusion/exclusion criteria. Following the 2-week enrollment period, eligible patients were randomized to either double-blind ONGLYZA (5 mg once daily) or double-blind matching placebo for 24 weeks. During the 24-week double-blind treatment period, patients were to receive metformin HCl and a sulfonylurea at the same constant dose ascertained during enrollment. Sulfonylurea dose could be down titrated once in the case of a major hypoglycemic event or recurring minor hypoglycemic events. In the absence of hypoglycemia, titration (up or down) of trial medication during the treatment period was prohibited.
ONGLYZA in combination with metformin HCl plus a sulfonylurea provided significant improvements in A1C and PPG compared with placebo in combination with metformin HCl plus a sulfonylurea (Table 11). The percentage of patients who discontinued for lack of glycemic control was 6% in the ONGLYZA group and 5% in the placebo group.
|
Efficacy Parameter |
ONGLYZA 5 mg
|
Placebo
|
|
Hemoglobin A1C (%) |
N=127 |
N=127 |
|
Baseline (mean) |
8.4 |
8.2 |
|
Change from baseline (adjusted mean†) |
−0.7 |
−0.1 |
|
Difference from placebo (adjusted mean†) |
−0.7‡ | |
|
95% Confidence Interval |
(−0.9, −0.5) | |
|
2-hour Postprandial Glucose (mg/dL) |
N=115 |
N=113 |
|
Baseline (mean) |
268 |
262 |
|
Change from baseline (adjusted mean†) |
−12 |
5 |
|
Difference from placebo (adjusted mean†) |
−17§ | |
|
95% Confidence Interval |
(−32, −2) | |
The change in fasting plasma glucose from baseline to Week 24 was also tested, but was not statistically significant. The percent of patients achieving an A1C <7% was 31% (39/127) with ONGLYZA in combination with metformin HCl plus a sulfonylurea compared to 9% (12/127) with placebo. Significance was not tested.
Add-on Combination Therapy with Metformin HCl plus an SGLT2 Inhibitor
A total of 315 patients with type 2 diabetes mellitus participated in this 24-week randomized, double-blind, placebo-controlled trial to evaluate the efficacy and safety of ONGLYZA added to dapagliflozin (an SGLT2 inhibitor) and metformin HCl in patients with a baseline of HbA1c ≥7% to ≤10.5%. The mean age of these patients was 54.6 years, 1.6% were 75 years or older and 52.7% were female. The population was 87.9% White, 6.3% Black or African American, 4.1% Asian, and 1.6% other races. At baseline the population had diabetes mellitus for an average of 7.7 years and a mean HbA1c of 7.9%. The mean eGFR at baseline was 93.4 mL/min/1.73 m2. Patients were required to be on a stable dose of metformin HCl (≥1,500 mg per day) for at least 8 weeks prior to enrollment. Eligible patients who completed the screening period entered the lead in treatment period, which included open-label metformin HCl and 10 mg dapagliflozin treatment. Following the lead-in period, eligible patients were randomized to ONGLYZA 5 mg (N=153) or placebo (N =162).
The group treated with add-on ONGLYZA had statistically significant greater reductions in HbA1c from baseline versus the group treated with placebo (see Table 12).
|
||
|
ONGLYZA 5 mg (N=153)† |
Placebo (N=162)† |
|
|
In combination with Dapagliflozin and Metformin HCl |
||
|
Hemoglobin A1C (%)‡ |
||
|
Baseline (mean) |
8.0 |
7.9 |
|
Change from baseline (adjusted mean§) 95% Confidence Interval |
−0.5 (−0.6, −0.4) |
−0.2 (−0.3, −0.1) |
|
Difference from placebo (adjusted mean) 95% Confidence Interval |
−0.4¶ (−0.5, −0.2) |
|
The known proportion of patients achieving HbA1c <7% at Week 24 was 35.3% in the saxagliptin treated group compared to 23.1% in the placebo treated group.
14.2 Renal Impairment
A total of 170 patients participated in a 12-week, randomized, double-blind, placebo-controlled trial conducted to evaluate the efficacy and safety of ONGLYZA 2.5 mg once daily compared with placebo in patients with type 2 diabetes mellitus and moderate (n=90) or severe (n=41) renal impairment or ESRD (n=39). In this trial, 98% of the patients were using background antidiabetic medications (75% were using insulin and 31% were using oral antidiabetic medications, mostly sulfonylureas).
After 12 weeks of treatment, ONGLYZA 2.5 mg provided significant improvement in A1C compared to placebo (Table 13). In the subgroup of patients with ESRD, ONGLYZA and placebo resulted in comparable reductions in A1C from baseline to Week 12. This finding is inconclusive because the trial was not adequately powered to show efficacy within specific subgroups of renal impairment.
After 12 weeks of treatment, the mean change in FPG was −12 mg/dL with ONGLYZA 2.5 mg and −13 mg/dL with placebo. Compared to placebo, the mean change in FPG with ONGLYZA was −12 mg/dL in the subgroup of patients with moderate renal impairment, −4 mg/dL in the subgroup of patients with severe renal impairment, and +44 mg/dL in the subgroup of patients with ESRD. These findings are inconclusive because the trial was not adequately powered to show efficacy within specific subgroups of renal impairment.
| Efficacy Parameter | ONGLYZA 2.5 mg
N=85 | Placebo
N=85 |
|---|---|---|
|
Hemoglobin A1C (%) |
N=81 |
N=83 |
|
Baseline (mean) |
8.4 |
8.1 |
|
Change from baseline (adjusted mean†) |
−0.9 |
−0.4 |
|
Difference from placebo (adjusted mean†) |
−0.4‡ | |
|
95% Confidence Interval |
(−0.7, −0.1) | |
14.3 Cardiovascular Safety Trial
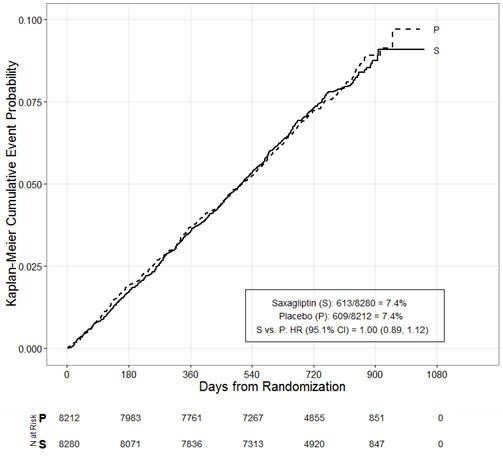
The cardiovascular risk of ONGLYZA was evaluated in SAVOR, a multicenter, multinational, randomized, double-blind trial comparing ONGLYZA (N=8280) to placebo (N=8212), both administered in combination with standard of care, in adult patients with type 2 diabetes mellitus at high risk for atherosclerotic cardiovascular disease. Of the randomized trial patients, 97.5% completed the trial, and the median duration of follow-up was approximately 2 years. The trial was event-driven, and patients were followed until a sufficient number of events were accrued.
Patients were at least 40 years of age, had A1C ≥6.5%, and multiple risk factors (21% of randomized patients) for cardiovascular disease (age ≥55 years for men and ≥60 years for women plus at least one additional risk factor of dyslipidemia, hypertension, or current cigarette smoking) or established (79% of the randomized patients) cardiovascular disease defined as a history of ischemic heart disease, peripheral vascular disease, or ischemic stroke. Overall, the use of diabetes medications was balanced across treatment groups (metformin HCl 69%, insulin 41%, sulfonylureas 40%, and TZDs 6%). The use of cardiovascular disease medications was also balanced (angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) 79%, statins 78%, aspirin 75%, beta-blockers 62%, and non-aspirin antiplatelet medications 24%).
The majority of patients were male (67%) and White (75%) with a mean age of 65 years. Approximately 16% of the population had moderate (estimated glomerular filtration rate [eGFR] ≥30 to ≤50 mL/min/1.73 m2) to severe (eGFR <30 mL/min/1.73 m2) renal impairment, and 13% had a prior history of heart failure. Patients had a median duration of type 2 diabetes mellitus of approximately 10 years, and a mean baseline A1C level of 8.0%. Approximately 5% of patients were treated with diet and exercise only at baseline. Overall, the use of diabetes medications was balanced across treatment groups (metformin HCl 69%, insulin 41%, sulfonylureas 40%, and TZDs 6%). The use of cardiovascular disease medications was also balanced (ACE inhibitors or ARBs 79%, statins 78%, aspirin 75%, beta-blockers 62%, and non-aspirin antiplatelet medications 24%).
The primary analysis in SAVOR was time to first occurrence of a Major Adverse Cardiac Event (MACE). A major adverse cardiac event in SAVOR was defined as a cardiovascular death or a nonfatal myocardial infarction (MI) or a nonfatal ischemic stroke. The trial was designed as a non-inferiority trial with a pre-specified risk margin of 1.3 for the hazard ratio of MACE and was also powered for a superiority comparison if non-inferiority was demonstrated.
The results of SAVOR, including the contribution of each component to the primary composite endpoint, are shown in Table 14. The incidence rate of MACE was similar in both treatment arms: 3.8 MACE per 100 patient-years on placebo vs. 3.8 MACE per 100 patient-years on ONGLYZA. The estimated hazard ratio of MACE associated with ONGLYZA relative to placebo was 1.00 with a 95.1% confidence interval of (0.89, 1.12). The upper bound of this confidence interval, 1.12, excluded a risk margin larger than 1.3.
|
ONGLYZA |
Placebo |
Hazard Ratio |
|||
|
|
|
|
|
|
|
Composite of first event of CV death, non-fatal MI or non-fatal ischemic stroke (MACE) |
|
|
|
| |
|
613 (7.4) |
3.8 |
609 (7.4) |
3.8 |
1.00 (0.89, 1.12) |
|
|
CV death |
245 (3.0) |
1.5 |
234 (2.8) |
1.4 | |
|
Non-fatal MI |
233 (2.8) |
1.4 |
260 (3.2) |
1.6 | |
|
Non-fatal ischemic stroke |
135 (1.6) |
0.8 |
115 (1.4) |
0.7 | |
The Kaplan-Meier-based cumulative event probability is presented in Figure 2 for time to first occurrence of the primary MACE composite endpoint by treatment arm. The curves for both ONGLYZA and placebo arms are close together throughout the duration of the trial. The estimated cumulative event probability is approximately linear for both arms, indicating that the incidence of MACE for both arms was constant over the trial duration.
Figure 2: Cumulative Percent of Time to First MACE
Vital status was obtained for 99% of patients in the trial. There were 798 deaths in the SAVOR trial. Numerically more patients (5.1%) died in the ONGLYZA group than in the placebo group (4.6%). The risk of deaths from all cause (Table 15) was not statistically different between the treatment groups (HR: 1.11; 95.1% CI: 0.96, 1.27).
|
ONGLYZA |
Placebo |
Hazard Ratio |
|||
|
Number of Patients (%) |
Rate per 100 PY |
Number of Patients (%) |
Rate per 100 PY |
(95.1% CI) |
|
|
N=8280 |
PY=16645.3 |
N=8212 |
PY=16531.5 | ||
|
All-cause mortality |
420 (5.1) |
2.5 |
378 (4.6) |
2.3 |
1.11 (0.96, 1.27) |
|
CV death |
269 (3.2) |
1.6 |
260 (3.2) |
1.6 | |
|
Non-CV death |
151 (1.8) |
0.9 |
118 (1.4) |
0.7 | |
16. How is Onglyza supplied
How Supplied
ONGLYZA tablets have markings on both sides and are available in the strengths and packages listed in Table 16.
| Tablet
Strength | Film-Coated Tablet
Color/Shape | Tablet
Markings | Package Size | NDC Code |
|---|---|---|---|---|
|
5 mg |
pink |
“5” on one side and “4215” on the reverse, in blue ink |
Bottles of 30 |
0310-6105-30 |
|
2.5 mg |
pale yellow to light yellow |
“2.5” on one side and “4214” on the reverse, in blue ink |
Bottles of 30 |
0310-6100-30 |
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Pancreatitis
Inform patients that acute pancreatitis has been reported during postmarketing use of ONGLYZA. Educate patients that persistent severe abdominal pain, sometimes radiating to the back, which may or may not be accompanied by vomiting, is the hallmark symptom of acute pancreatitis. Instruct patients to promptly discontinue ONGLYZA and contact their health care provider if persistent severe abdominal pain occurs [see Warnings and Precautions (5.1)].
Heart Failure
Inform patients of the signs and symptoms of heart failure. Before initiating ONGLYZA, ask patients if they have a history of heart failure or other risk factors for heart failure including moderate to severe renal impairment. Instruct patients to contact their health care provider as soon as possible if they experience symptoms of heart failure, including increasing shortness of breath, rapid increase in weight or swelling of the feet [see Warnings and Precautions (5.2)].
Hypoglycemia with Concomitant Use with Insulin or Insulin Secretagogues
Inform patients that hypoglycemia can occur, particularly when insulin or an insulin secretagogue is used in combination with ONGLYZA. Educate patients about the risks, symptoms and appropriate management of hypoglycemia [see Warnings and Precautions (5.3)].
Hypersensitivity Reactions
Patients should be informed that serious allergic (hypersensitivity) reactions, such as angioedema, anaphylaxis, and exfoliative skin conditions, have been reported during postmarketing use of ONGLYZA. If symptoms of these allergic reactions (such as rash, skin flaking or peeling, urticaria, swelling of the skin, or swelling of the face, lips, tongue, and throat that may cause difficulty in breathing or swallowing) occur, patients must stop taking ONGLYZA and seek medical advice promptly [see Warnings and Precautions (5.4)].
Severe and Disabling Arthralgia
Inform patients that severe and disabling joint pain may occur with this class of drugs. The time to onset of symptoms can range from one day to years. Instruct patients to seek medical advice if severe joint pain occurs [see Warnings and Precautions (5.5)].
Bullous Pemphigoid
Inform patients that bullous pemphigoid may occur with this class of drugs. Instruct patients to seek medical advice if blisters or erosions occur [see Warnings and Precautions (5.6)].
Missed Dose
If a dose is missed, advise patients to take ONGLYZA as soon as they remember unless it is time for their next dose. Instruct patients not to take two doses of ONGLYZA at the same time.
Administration Instructions
Advise patients not to cut, crush, or chew ONGLYZA tablets.
|
MEDICATION GUIDE ONGLYZA® (on-GLY-zah) (saxagliptin) tablets, for oral use |
|
|
What is the most important information I should know about ONGLYZA? Serious side effects can happen to people taking ONGLYZA, including: 1) Inflammation of the pancreas (pancreatitis) which may be severe and lead to death. Certain medical problems make you more likely to get pancreatitis. Before you start taking ONGLYZA: Tell your healthcare provider if you have ever had |
|
|
|
|
Stop taking ONGLYZA and contact your healthcare provider right away if you have pain in your stomach area (abdomen) that is severe and will not go away. The pain may be felt going from your abdomen through to your back. The pain may happen with or without vomiting. These may be symptoms of pancreatitis. 2) Heart failure. Heart failure means your heart does not pump blood well enough. Before you start taking ONGLYZA: Tell your healthcare provider if you
Contact your healthcare provider right away if you have any of the following symptoms: |
|
|
|
|
These may be symptoms of heart failure. |
|
|
What is ONGLYZA?
It is not known if ONGLYZA is safe and effective in children. |
|
|
Who should not take ONGLYZA? Do not take ONGLYZA if you:
|
|
|
|
|
If you have these symptoms, stop taking ONGLYZA and contact your healthcare provider right away. |
|
|
Before taking ONGLYZA, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Know the medicines you take. Keep a list of your medicines and show it to your healthcare provider and pharmacist when you get a new medicine. ONGLYZA may affect the way other medicines work, and other medicines may affect how ONGLYZA works. Contact your healthcare provider if you will be starting or stopping certain other types of medicines, such as antibiotics, or medicines that treat fungus or HIV/AIDS, because your dose of ONGLYZA might need to be changed. |
|
|
How should I take ONGLYZA?
|
|
|
What are the possible side effects of ONGLYZA? ONGLYZA can cause serious side effects, including:
If you have these symptoms, stop taking ONGLYZA and contact your healthcare provider right away.
The most common side effects of ONGLYZA include:
These are not all of the possible side effects of ONGLYZA. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|
|
How should I store ONGLYZA? Store ONGLYZA between 68°F to 77°F (20°C to 25°C). Keep ONGLYZA and all medicines out of the reach of children. |
|
|
General information about the use of ONGLYZA Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use ONGLYZA for a condition for which it was not prescribed. Do not give ONGLYZA to other people, even if they have the same symptoms you have. It may harm them. You can ask your healthcare provider for additional information about ONGLYZA that is written for health professionals. |
|
|
What are the ingredients of ONGLYZA? Active ingredient: saxagliptin Inactive ingredients: croscarmellose sodium, lactose monohydrate, magnesium stearate, and microcrystalline cellulose. In addition, the film coating contains the following inactive ingredients: iron oxides, polyethylene glycol polyvinyl alcohol, talc, and titanium dioxide. ONGLYZA is a registered trademark of the AstraZeneca group of companies. Distributed by: AstraZeneca Pharmaceuticals LP Wilmington, DE 19850. For more information, go to www.ONGLYZA.com or call 1 800 ONGLYZA. |
|
|
This Medication Guide has been approved by the U.S. Food and Drug Administration. 10/2024 |
|
| ONGLYZA
saxagliptin tablet, film coated |
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| ONGLYZA
saxagliptin tablet, film coated |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - AstraZeneca Pharmaceuticals LP (054743190) |
| Registrant - AstraZeneca PLC (230790719) |
Frequently asked questions
More about Onglyza (saxagliptin)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (15)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Generic availability
- FDA approval history
- Drug class: dipeptidyl peptidase 4 inhibitors
- Breastfeeding
- En español