Nubain: Package Insert / Prescribing Info
Package insert / product label
Generic name: nalbuphine hydrochloride
Dosage form: Injection
Drug class: Opioids (narcotic analgesics)
Medically reviewed by Drugs.com. Last updated on Mar 25, 2025.
The Nubain brand name has been discontinued in the U.S. If generic versions of this product have been approved by the FDA, there may be generic equivalents available.
On This Page
Nubain Description
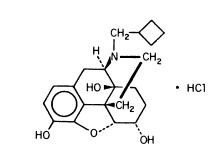
NUBAIN (nalbuphine hydrochloride) is a synthetic opioid agonist-antagonist analgesic of the phenanthrene series. It is chemically related to both the widely used opioid antagonist, naloxone, and the potent opioid analgesic, oxymorphone. Chemically nalbuphine hydrochloride is 17-(cyclobutylmethyl)-4,5α-epoxymorphinan-3,6α,14-triol hydrochloride. Nalbuphine hydrochloride molecular weight is 393.91 and is soluble in H2O (35.5 mg/mL @ 25ºC) and ethanol (0.8%); insoluble in CHCl3 and ether. Nalbuphine hydrochloride has pKa values of 8.71 and 9.96. The molecular formula is C21H27NO4 · HCl. The structural formula is:
NUBAIN is a sterile solution suitable for subcutaneous, intramuscular, or intravenous injection. NUBAIN is available in two concentrations, 10 mg and 20 mg of nalbuphine hydrochloride per mL. Both strengths in 10 mL vials contain 0.94% sodium citrate hydrous, 1.26% citric acid anhydrous, and 0.2% of a 9:1 mixture of methylparaben and propylparaben as preservatives; pH is adjusted, if necessary, to 3.5 to 3.7 with hydrochloric acid. The 10 mg/mL strength contains 0.2% sodium chloride.
NUBAIN is also available in ampuls in a sterile, paraben-free formulation in two concentrations, 10 mg and 20 mg of nalbuphine hydrochloride per mL. One mL of each strength contains 0.94% sodium citrate hydrous, and 1.26% citric acid anhydrous; pH is adjusted, if necessary, to 3.5 to 3.7 with hydrochloric acid. The 10 mg/mL strength contains 0.2% sodium chloride.
Nubain - Clinical Pharmacology
NUBAIN is a potent analgesic. Its analgesic potency is essentially equivalent to that of morphine on a milligram basis. Receptor studies show that NUBAIN binds to mu, kappa, and delta receptors, but not to sigma receptors. NUBAIN is primarily a kappa agonist/partial mu antagonist analgesic.
The onset of action of NUBAIN occurs within 2 to 3 minutes after intravenous administration, and in less than 15 minutes following subcutaneous or intramuscular injection. The plasma half-life of nalbuphine is 5 hours, and in clinical studies the duration of analgesic activity has been reported to range from 3 to 6 hours.
The opioid antagonist activity of NUBAIN is one-fourth as potent as nalorphine and 10 times that of pentazocine.
NUBAIN may produce the same degree of respiratory depression as equianalgesic doses of morphine. However, NUBAIN exhibits a ceiling effect such that increases in dose greater than 30 mg do not produce further respiratory depression in the absence of other CNS active medications affecting respiration.
NUBAIN by itself has potent opioid antagonist activity at doses equal to or lower than its analgesic dose. When administered following or concurrent with mu agonist opioid analgesics (e.g., morphine, oxymorphone, fentanyl), NUBAIN may partially reverse or block opioid-induced respiratory depression from the mu agonist analgesic. NUBAIN may precipitate withdrawal in patients dependent on opioid drugs. NUBAIN should be used with caution in patients who have been receiving mu opioid analgesics on a regular basis.
Indications and Usage for Nubain
NUBAIN is indicated for the relief of moderate to severe pain. NUBAIN can also be used as a supplement to balanced anesthesia, for preoperative and postoperative analgesia, and for obstetrical analgesia during labor and delivery.
Contraindications
NUBAIN should not be administered to patients who are hypersensitive to nalbuphine hydrochloride, or to any of the other ingredients in NUBAIN.
Warnings
NUBAIN should be administered as a supplement to general anesthesia only by persons specifically trained in the use of intravenous anesthetics and management of the respiratory effects of potent opioids.
Naloxone, resuscitative and intubation equipment and oxygen should be readily available.
Drug Abuse
Caution should be observed in prescribing NUBAIN for emotionally unstable patients, or for individuals with a history of opioid abuse. Such patients should be closely supervised when long-term therapy is contemplated (see DRUG ABUSE AND DEPENDENCE).
Use in Ambulatory Patients
NUBAIN may impair the mental or physical abilities required for the performance of potentially dangerous tasks such as driving a car or operating machinery. Therefore, NUBAIN should be administered with caution to ambulatory patients who should be warned to avoid such hazards.
Use in Emergency Procedures
Maintain patient under observation until recovered from NUBAIN effects that would affect driving or other potentially dangerous tasks.
Use in Pregnancy (Other Than Labor)
Severe fetal bradycardia has been reported when NUBAIN is administered during labor. Naloxone may reverse these effects. Although there are no reports of fetal bradycardia earlier in pregnancy, it is possible that this may occur. This drug should be used in pregnancy only if clearly needed, if the potential benefit outweighs the risk to the fetus, and if appropriate measures such as fetal monitoring are taken to detect and manage any potential adverse effect on the fetus.
Use During Labor and Delivery
The placental transfer of nalbuphine is high, rapid, and variable with a maternal to fetal ratio ranging from 1:0.37 to 1:6. Fetal and neonatal adverse effects that have been reported following the administration of nalbuphine to the mother during labor include fetal bradycardia, respiratory depression at birth, apnea, cyanosis, and hypotonia. Some of these events have been life-threatening. Maternal administration of naloxone during labor has normalized these effects in some cases. Severe and prolonged fetal bradycardia has been reported. Permanent neurological damage attributed to fetal bradycardia has occurred. A sinusoidal fetal heart rate pattern associated with the use of nalbuphine has also been reported. NUBAIN should be used during labor and delivery only if clearly indicated and only if the potential benefit outweighs the risk to the infant. Newborns should be monitored for respiratory depression, apnea, bradycardia and arrhythmias if NUBAIN has been used.
Head Injury and Increased Intracranial Pressure
The possible respiratory depressant effects and the potential of potent analgesics to elevate cerebrospinal fluid pressure (resulting from vasodilation following CO2 retention) may be markedly exaggerated in the presence of head injury, intracranial lesions or a pre-existing increase in intracranial pressure. Furthermore, potent analgesics can produce effects which may obscure the clinical course of patients with head injuries. Therefore, NUBAIN should be used in these circumstances only when essential, and then should be administered with extreme caution.
Interaction with Other Central Nervous System Depressants
Although NUBAIN possesses opioid antagonist activity, there is evidence that in nondependent patients it will not antagonize an opioid analgesic administered just before, concurrently, or just after an injection of NUBAIN. Therefore, patients receiving an opioid analgesic, general anesthetics, phenothiazines, or other tranquilizers, sedatives, hypnotics, or other CNS depressants (including alcohol) concomitantly with NUBAIN may exhibit an additive effect. When such combined therapy is contemplated, the dose of one or both agents should be reduced.
Precautions
General
Impaired Respiration
At the usual adult dose of 10 mg/70 kg, NUBAIN causes some respiratory depression approximately equal to that produced by equal doses of morphine. However, in contrast to morphine, respiratory depression is not appreciably increased with higher doses of NUBAIN. Respiratory depression induced by NUBAIN can be reversed by NARCAN® (naloxone hydrochloride) when indicated. NUBAIN should be administered with caution at low doses to patients with impaired respiration (e.g., from other medication, uremia, bronchial asthma, severe infection, cyanosis, or respiratory obstructions).
Impaired Renal or Hepatic Function
Because NUBAIN is metabolized in the liver and excreted by the kidneys, NUBAIN should be used with caution in patients with renal or liver dysfunction and administered in reduced amounts.
Myocardial Infarction
As with all potent analgesics, NUBAIN should be used with caution in patients with myocardial infarction who have nausea or vomiting.
Information for Patients
Patients should be advised of the following information:
- NUBAIN is associated with sedation and may impair mental and physical abilities required for the performance of potentially dangerous tasks such as driving a car or operating machinery.
- NUBAIN is to be used as prescribed by a physician. Dose or frequency should not be increased without first consulting with a physician since NUBAIN may cause psychological or physical dependence.
- The use of NUBAIN with other opioids can cause signs and symptoms of withdrawal.
- Abrupt discontinuation of NUBAIN after prolonged usage may cause signs and symptoms of withdrawal.
Laboratory Tests
NUBAIN may interfere with enzymatic methods for the detection of opioids depending on the specificity/sensitivity of the test. Consult the test manufacturer for specific details.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long term carcinogenicity studies were performed in rats (24 months) and mice (19 months) by oral administration at doses up to 200 mg/kg (1180 mg/m2) and 200 mg/kg (600 mg/m2) per day, respectively. There was no evidence of an increase in tumors in either species related to NUBAIN administration. The maximum recommend human dose (MRHD) in a day is 160 mg subcutaneously, intramuscularly or intravenously, or approximately 100 mg/m2/day for a 60 kg subject.
Mutagenesis
NUBAIN did not have mutagenic activity in the AMES test with four bacterial strains, in the Chinese Hamster Ovary HGPRT assays or in the Sister Chromatids Exchange Assay. However, NUBAIN induced an increased frequency of mutation in the mouse lymphoma assay. Clastogenic activity was not observed in the mouse micronucleus test of the cytogenicity bone marrow assay in rats.
Usage in Pregnancy
Teratogenic Effects: Pregnancy Category B
Reproduction studies have been performed in rats by subcutaneous administration of nalbuphine up to 100 mg/kg/day, or 590 mg/m2/day which is approximately 6 times the MRHD, and in rabbits by intravenous administration of nalbuphine up to 32 mg/kg/day, or 378 mg/m2/day which is approximately 4 times the MRHD. The results did not reveal evidence of developmental toxicity, including teratogenicity, or harm to the fetus. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Non-teratogenic Effects
Neonatal body weight and survival rates were reduced at birth and during lactation when nalbuphine was subcutaneously administered to female and male rats prior to mating and throughout gestation and lactation or to pregnant rats during the last third of gestation and throughout lactation at doses approximately 4 times the maximum recommended human dose.
Adverse Reactions/Side Effects
The most frequent adverse reaction in 1066 patients treated in clinical studies with NUBAIN was sedation 381 (36%).
Less frequent reactions were: sweaty/clammy 99 (9%), nausea/vomiting 68 (6%), dizziness/vertigo 58 (5%), dry mouth 44 (4%), and headache 27 (3%).
Other adverse reactions which occurred (reported incidence of 1% or less) were:
CNS Effects
Nervousness, depression, restlessness, crying, euphoria, floating, hostility, unusual dreams, confusion, faintness, hallucinations, dysphoria, feeling of heaviness, numbness, tingling, unreality. The incidence of psychotomimetic effects, such as unreality, depersonalization, delusions, dysphoria and hallucinations has been shown to be less than that which occurs with pentazocine.
Allergic Reactions
Anaphylactic/anaphylactoid and other serious hypersensitivity reactions have been reported following the use of nalbuphine and may require immediate, supportive medical treatment. These reactions may include shock, respiratory distress, respiratory arrest, bradycardia, cardiac arrest, hypotension, or laryngeal edema. Some of these allergic reactions may be life-threatening. Other allergic-type reactions reported include stridor, bronchospasm, wheezing, edema, rash, pruritus, nausea, vomiting, diaphoresis, weakness, and shakiness.
Events Observed during Post-marketing Surveillance of NUBAIN
Due to the nature and limitations of spontaneous reporting, causality has not been established for the following adverse events received for NUBAIN: abdominal pain, pyrexia, depressed level or loss of consciousness, somnolence, tremor, anxiety, pulmonary edema, agitation, seizures, and injection site reactions such as pain, swelling, redness, burning, and hot sensations. Death has been reported from severe allergic reactions to NUBAIN treatment. Fetal death has been reported where mothers received NUBAIN during labor and delivery.
Related/similar drugs
Drug Abuse and Dependence
There have been reports of abuse and dependence associated with NUBAIN among health care providers, patients and bodybuilders. There have been reported instances of psychological and physical dependence and tolerance in patients abusing NUBAIN. Individuals with a prior history of opioid or other substance abuse or dependence may be at greater risk in responding to reinforcing properties of NUBAIN.
Abrupt discontinuation of NUBAIN following prolonged use has been followed by symptoms of opioid withdrawal, i.e., abdominal cramps, nausea and vomiting, rhinorrhea, lacrimation, restlessness, anxiety, elevated temperature and piloerection.
Overdosage
The immediate intravenous administration an opiate antagonist such as naloxone or nalmefene is a specific antidote. Oxygen, intravenous fluids, vasopressors and other supportive measures should be used as indicated.
The administration of single doses of 72 mg of NUBAIN subcutaneously to eight normal subjects has been reported to have resulted primarily in symptoms of sleepiness and mild dysphoria.
Nubain Dosage and Administration
The usual recommended adult dose is 10 mg for a 70 kg individual, administered subcutaneously, intramuscularly or intravenously; this dose may be repeated every 3 to 6 hours as necessary. Dosage should be adjusted according to the severity of the pain, physical status of the patient, and other medications which the patient may be receiving. (See Interaction with Other Central Nervous System Depressants under WARNINGS). In non-tolerant individuals, the recommended single maximum dose is 20 mg, with a maximum total daily dose of 160 mg. The use of NUBAIN as a supplement to balanced anesthesia requires larger doses than those recommended for analgesia. Induction doses of NUBAIN range from 0.3 mg/kg to 3 mg/kg intravenously to be administered over a 10 to 15 minute period with maintenance doses of 0.25 to 0.5 mg/kg in single intravenous administrations as required. The use of NUBAIN may be followed by respiratory depression which can be reversed with the opioid antagonist NARCAN® (naloxone hydrochloride).
NUBAIN is physically incompatible with nafcillin and keterolac.
Patients Dependent on Opioids
Patients who have been taking opioids chronically may experience withdrawal symptoms upon the administration of NUBAIN. If unduly troublesome, opioid withdrawal symptoms can be controlled by the slow intravenous administration of small increments of morphine, until relief occurs. If the previous analgesic was morphine, meperidine, codeine, or other opioid with similar duration of activity, one-fourth of the anticipated dose of NUBAIN can be administered initially and the patient observed for signs of withdrawal, i.e., abdominal cramps, nausea and vomiting, lacrimation, rhinorrhea, anxiety, restlessness, elevation of temperature or piloerection. If untoward symptoms do not occur, progressively larger doses may be tried at appropriate intervals until the desired level of analgesia is obtained with NUBAIN.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
How is Nubain supplied
NUBAIN® (nalbuphine hydrochloride) injection for intramuscular, subcutaneous, or intravenous use is a sterile solution available in:
NDC 63481-508-05 (sulfite-free) 10 mg/mL, 10 mL multiple dose vials (box of 1)
NDC 63481-432-10 (sulfite/paraben-free) 10 mg/mL, 1 mL ampuls (box of 10)
NDC 63481-509-05 (sulfite-free) 20 mg/mL, 10 mL multiple dose vials (box of 1)
NDC 63481-433-10 (sulfite/paraben-free) 20 mg/mL, 1 mL ampuls (box of 10)
Store at 25°C (77°F); excursions permitted to 15°-30°C (59°-86°F). [See USP Controlled Room Temperature.] Protect from excessive light. Store in carton until contents have been used.
Manufactured for:
Endo Pharmaceuticals Inc.
Chadds Ford, Pennsylvania 19317
Manufactured by:
Bristol-Myers Squibb Holdings Pharma, Ltd.
Manati, Puerto Rico 00674 USA
NUBAIN® is a Registered Trademark of Endo Pharmaceuticals Inc.
NARCAN® is a Registered Trademark of Endo Pharmaceuticals Inc.
Copyright © Endo Pharmaceuticals Inc. 2005
Printed in U.S.A. 51-022542-01/August, 2005
| NUBAIN
nalbuphine hydrochloride injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| NUBAIN
nalbuphine hydrochloride injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| NUBAIN
nalbuphine hydrochloride injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| NUBAIN
nalbuphine hydrochloride injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Endo Pharmaceuticals Inc. |
More about Nubain (nalbuphine)
- Check interactions
- Compare alternatives
- Reviews (28)
- Side effects
- Dosage information
- During pregnancy
- Drug class: Opioids (narcotic analgesics)
- Breastfeeding
