Mucinex Maximum Strength: Package Insert / Prescribing Info
Package insert / product label
Generic name: guaifenesin
Dosage form: tablet, extended release
Drug class: Expectorants
Medically reviewed by Drugs.com. Last updated on Aug 14, 2025.
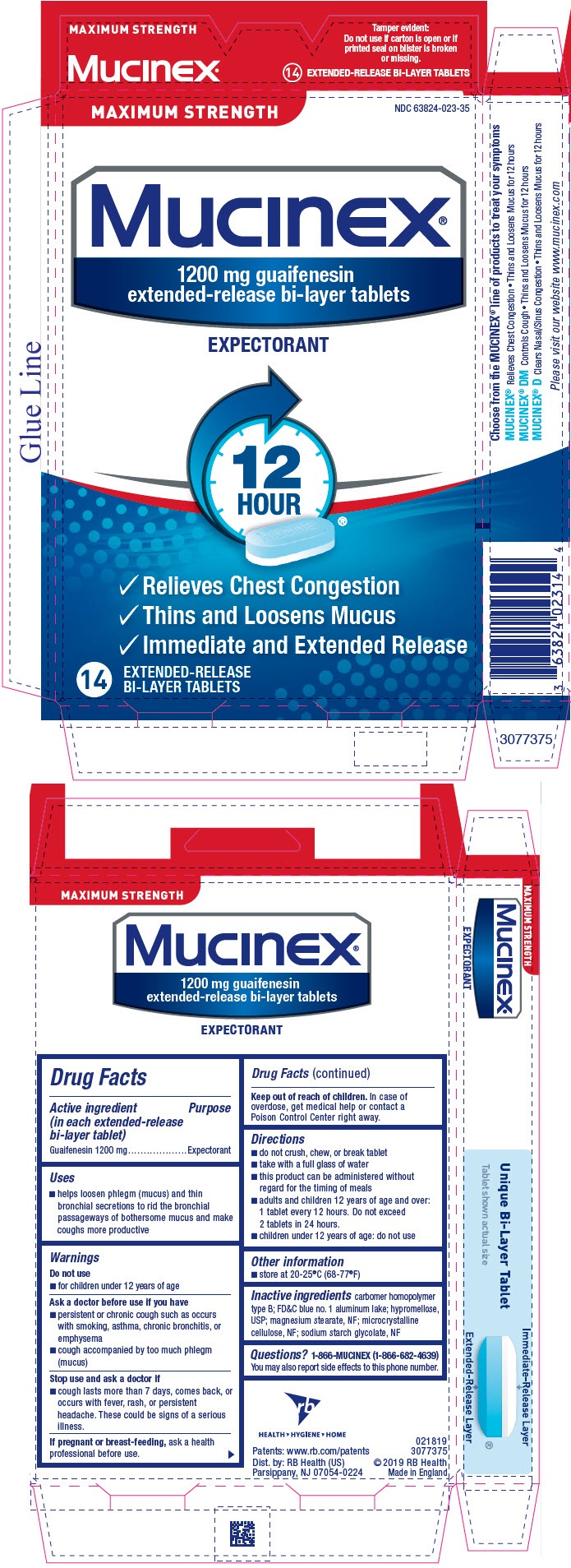
Active ingredient (in each extended-release bi-layer tablet)
Guaifenesin 1200 mg
Indications and Usage for Mucinex Maximum Strength
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
Warnings
Do not use
- for children under 12 years of age
Ask a doctor before use if you have
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- cough accompanied by too much phlegm (mucus)
Stop use and ask a doctor if
- cough lasts more than 7 days, comes back, or occurs with fever, rash, or persistent headache. These could be signs of a serious illness.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Mucinex Maximum Strength Dosage and Administration
- do not crush, chew, or break tablet
- take with a full glass of water
- this product can be administered without regard for the timing of meals
- adults and children 12 years of age and over: 1 tablet every 12 hours. Do not exceed 2 tablets in 24 hours.
- children under 12 years of age: do not use
Related/similar drugs
Storage and Handling
- store at 20-25°C (68-77°F)
Inactive ingredients
carbomer homopolymer type B; FD&C blue no. 1 aluminum lake; hypromellose, USP; magnesium stearate, NF; microcrystalline cellulose, NF; sodium starch glycolate, NF
Questions?
1-866-MUCINEX (1-866-682-4639) You may also report side effects to this phone number.
Dist. by: RB Health (US)
Parsippany, NJ 07054-0224
Made in England
PRINCIPAL DISPLAY PANEL - 14 Tablet Blister Pack Carton
NDC 63824-023-35
MAXIMUM STRENGTH
Mucinex®
1200 mg guaifenesin
extended-release bi-layer tablets
EXPECTORANT
12
HOUR®
- 🗸
- Relieves Chest Congestion
- 🗸
- Thins and Loosens Mucus
- 🗸
- Immediate and Extended Release
14
EXTENDED-RELEASE
BI-LAYER TABLETS
MUCINEX
MAXIMUM STRENGTH
guaifenesin tablet, extended release |
|
|
|
|
|
|
|
|
|
|
|
|
|
Frequently asked questions
More about Mucinex Maximum Strength (guaifenesin)
Patient resources
Professional resources
Other formulations
Related treatment guides
Medical Disclaimer