Enalapril Oral Solution: Package Insert / Prescribing Info
Package insert / product label
Generic name: enalapril maleate
Dosage form: oral solution
Drug class: Angiotensin Converting Enzyme Inhibitors
Medically reviewed by Drugs.com. Last updated on Oct 6, 2024.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Drug Interactions
- Use In Specific Populations
- Overdosage
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
ENALAPRIL MALEATE oral solution
Initial U.S. Approval: 1985
Indications and Usage for Enalapril Oral Solution
Enalapril Oral Solution Dosage and Administration
Heart Failure: Initiate at 2.5 mg twice daily. Titrate up to 20 mg twice daily as tolerated. ( 2.2)
Asymptomatic Left Ventricular Dysfunction: Initiate at 2.5 mg twice daily. Titrate up to 10 mg twice daily. ( 2.3)
Enalapril maleate oral solution is a ready-to-use solution intended for oral use only.
Dosage Forms and Strengths
Enalapril maleate oral solution is a ready-to-use oral solution: 1 mg/mL enalapril maleate, USP. ( 3)
Contraindications
Warnings and Precautions
Adverse Reactions/Side Effects
- The most common adverse reactions for patients treated for heart failure (>6%) were hypotension and dizziness. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Bionpharma Inc. at 1-888-235-BION or 1-888-235-2466 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- In patients who are elderly, volume-depleted (as on diuretic therapy), or with compromised renal function, use with NSAIDs, including selective COX-2 inhibitors, may result in deterioration of renal function, including renal failure. Monitor renal function periodically. ( 7.1)
- Dual inhibition of the renin-angiotensin system: Increased risk of renal impairment, hypotension and hyperkalemia. ( 7.2)
- Avoid potassium sparing agents in patients with heart failure. ( 7.3)
- Monitor serum lithium levels frequently. ( 7.4)
Use In Specific Populations
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 7/2024
Full Prescribing Information
1. Indications and Usage for Enalapril Oral Solution
1.2 Heart Failure
Enalapril maleate oral solution is indicated for the treatment of symptomatic heart failure, usually in combination with diuretics and digitalis. In these patients, enalapril maleate oral solution increases survival and decreases the frequency of hospitalization.
1.3 Asymptomatic Left Ventricular Dysfunction
In clinically stable asymptomatic patients with left ventricular dysfunction (ejection fraction ≤35 percent), enalapril maleate oral solution decreases the rate of development of overt heart failure and decreases the incidence of hospitalization for heart failure.
2. Enalapril Oral Solution Dosage and Administration
2.2 Heart Failure
The recommended initial dose is 2.5 mg twice a day titrated up to a maximum of 20 mg twice a day, as tolerated. Doses are usually given in combination with diuretics and digitalis.
In patients with hyponatremia (serum sodium less than 130 mEq/L) or serum creatinine greater than 1.6 mg/dL, the recommended initial dose is 2.5 mg once daily.
Diuretic dose may need to be adjusted to minimize hypovolemia and hypotension. The appearance of hypotension after the initial dose of enalapril maleate oral solution does not preclude subsequent careful dose titration with the drug, following effective management of the hypotension.
3. Dosage Forms and Strengths
Enalapril maleate oral solution is a ready-to-use oral solution that contains 1 mg/mL of enalapril maleate, USP. It is a clear, colorless solution with a mixed berry flavor packaged in a 150 mL white, round, high-density polyethylene bottle with a white, polypropylene, child-resistant cap and tamper-evident seal. Each bottle contains 150 mL.
4. Contraindications
Enalapril is contraindicated in patients with:
- a history of angioedema or hypersensitivity related to previous treatment with an angiotensin converting enzyme (ACE) inhibitor. [see Warnings and Precautions ( 5.2)]
- hereditary or idiopathic angioedema. [see Warnings and Precautions ( 5.2)]
Do not co-administer aliskiren with enalapril in patients with diabetes [see Drug Interactions ( 7.2)] .
Enalapril is contraindicated in combination with a neprilysin inhibitor (e.g., sacubitril). Do not administer enalapril within 36 hours of switching to or from sacubitril/valsartan, a neprilysin inhibitor [see Warnings and Precautions ( 5.2)] .
5. Warnings and Precautions
5.1 Fetal Toxicity
Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death.
Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue enalapril as soon as possible [see Use in Specific Populations ( 8.1)] .
5.2 Angioedema and Anaphylactoid Reactions
Angioedema
Head and Neck Angioedema
Angioedema of the face, extremities, lips, tongue, glottis and/or larynx, including some fatal reactions, have occurred in patients treated with angiotensin converting enzyme inhibitors, including enalapril, at any time during treatment. Patients with involvement of the tongue, glottis or larynx are likely to experience airway obstruction, especially those with a history of airway surgery. Enalapril should be promptly discontinued and appropriate therapy and monitoring should be provided until complete and sustained resolution of signs and symptoms of angioedema has occurred.
Patients with a history of angioedema unrelated to ACE inhibitor therapy may be at increased risk of angioedema while receiving an ACE inhibitor [see Contraindications ( 4)] . ACE inhibitors have been associated with a higher rate of angioedema in black than in non-black patients.
Patients receiving co-administration of ACE inhibitor and mTOR (mammalian target of rapamycin) inhibitor (e.g., temsirolimus, sirolimus, everolimus) therapy or a neprilysin inhibitor may be at increased risk for angioedema [see Drug Interactions ( 7.6, 7.7)].
Intestinal Angioedema
Intestinal angioedema has occurred in patients treated with ACE inhibitors. These patients presented with abdominal pain (with or without nausea or vomiting); in some cases there was no prior history of facial angioedema and C-1 esterase levels were normal. In some cases, the angioedema was diagnosed by procedures including abdominal CT scan or ultrasound, or at surgery, and symptoms resolved after stopping the ACE inhibitor.
Anaphylactoid Reactions
Anaphylactoid Reactions during Desensitization
Two patients undergoing desensitizing treatment with hymenoptera venom while receiving ACE inhibitors sustained life-threatening anaphylactoid reactions.
Anaphylactoid Reactions during Dialysis
Sudden and potentially life-threatening anaphylactoid reactions have occurred in some patients dialyzed with high-flux membranes and treated concomitantly with an ACE inhibitor. In such patients, dialysis must be stopped immediately, and aggressive therapy for anaphylactoid reactions must be initiated. Symptoms have not been relieved by antihistamines in these situations. In these patients, consideration should be given to using a different type of dialysis membrane or a different class of antihypertensive agent. Anaphylactoid reactions have also been reported in patients undergoing low-density lipoprotein apheresis with dextran sulfate absorption.
5.3 Hypotension
Enalapril can cause symptomatic hypotension, sometimes complicated by oliguria, progressive azotemia, acute renal failure or death. Patients at risk of excessive hypotension include those with the following conditions or characteristics: heart failure with systolic blood pressure below 100 mmHg, ischemic heart disease, cerebrovascular disease, hyponatremia, high dose diuretic therapy, renal dialysis, or severe volume and/or salt depletion of any etiology.
In these patients, enalapril should be started under very close medical supervision and such patients should be followed closely for the first two weeks of treatment and whenever the dose of enalapril and/or diuretic is increased.
Symptomatic hypotension is also possible in patients with severe aortic stenosis or hypertrophic cardiomyopathy.
Surgery/Anesthesia
In patients undergoing major surgery or during anesthesia with agents that produce hypotension, enalapril may block angiotensin II formation secondary to compensatory renin release. If hypotension occurs and is considered to be through this mechanism, it can be corrected by volume expansion.
5.4 Hepatic Failure
Rarely, ACE inhibitors have been associated with a syndrome that starts with cholestatic jaundice and progresses to fulminant hepatic necrosis, and (sometimes) death. The mechanism of this syndrome is not understood. Patients receiving ACE inhibitors who develop jaundice or marked elevations of hepatic enzymes should discontinue the ACE inhibitor and receive appropriate medical follow-up.
5.5 Impaired Renal Function
Monitor renal function in patients treated with enalapril. Changes in renal function including acute renal failure can be caused by drugs that inhibit the renin-angiotensin system. Patients whose renal function may depend in part on the activity of the renin-angiotensin system (e.g., patients with renal artery stenosis, chronic kidney disease, severe congestive heart failure, post-myocardial infarction or volume depletion) may be at particular risk of developing acute renal failure on enalapril. Consider withholding or discontinuing therapy in patients who develop a clinically significant decrease in renal function on enalapril [see Adverse Reactions ( 6.2), Drug Interactions ( 7.2, 7.3)] .
5.6 Hyperkalemia
Serum potassium should be monitored in patients receiving enalapril. Drugs that inhibit the renin-angiotensin system can cause hyperkalemia. Risk factors for the development of hyperkalemia include renal insufficiency, diabetes mellitus, and the concomitant use of potassium-sparing diuretics, potassium supplements and/or potassium-containing salt substitutes [see Drug Interactions ( 7.3)] .
6. Adverse Reactions/Side Effects
The following adverse reactions are described elsewhere:
- Angioedema [see Warnings and Precautions ( 5.2)]
- Hypotension [see Warnings and Precautions ( 5.3)]
- Hepatic failure [see Warnings and Precautions ( 5.4)]
- Renal impairment [see Warnings and Precautions ( 5.5)]
- Hyperkalemia [see Warnings and Precautions ( 5.6)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Enalapril has been evaluated for safety in more than 10,000 patients, including over 1,000 patients treated for one year or more.
In clinical trials, discontinuation of therapy for clinical adverse experiences was required in 5.7% of patients with heart failure.
Heart Failure
In patients treated for heart failure, there was an increased incidence of hypotension 6.7 percent versus 0.6 percent in placebo and dizziness 7.9 percent versus 0.6 percent in placebo.
6.2 Other Adverse Reactions from Clinical Studies or Postmarketing Experience
The following adverse reactions have been reported in clinical studies or postmarketing experience with enalapril. Because some of these reactions were reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Other serious clinical adverse experiences occurring since the drug was marketed or adverse experiences occurring in 0.5 to 1.0% of patients with hypertension or heart failure in clinical trials are listed below and, within each category, are in order of decreasing severity.
Cardiovascular:Cardiac arrest; myocardial infarction or cerebrovascular accident, possibly secondary to excessive hypotension in high risk patients [see Warnings and Precautions ( 5.3)] ; pulmonary embolism and infarction; pulmonary edema; rhythm disturbances, including atrial tachycardia and bradycardia; atrial fibrillation; palpitation; Raynaud's phenomenon.
Digestive:Ileus, pancreatitis, melena, anorexia, dyspepsia, constipation, glossitis, stomatitis, dry mouth.
Hematologic:Rare cases of neutropenia, thrombocytopenia, and bone marrow depression.
Musculoskeletal:Muscle cramps.
Nervous/Psychiatric:Depression, confusion, ataxia, somnolence, insomnia, nervousness, peripheral neuropathy (e.g., paresthesia, dysesthesia), dream abnormality.
Respiratory:Bronchospasm, rhinorrhea, sore throat and hoarseness, asthma, upper respiratory infection, pulmonary infiltrates, eosinophilic pneumonitis.
Skin:Exfoliative dermatitis, toxic epidermal necrolysis, Stevens-Johnson syndrome, pemphigus, herpes zoster, erythema multiforme, urticaria, pruritus, alopecia, flushing, diaphoresis, photosensitivity.
Special Senses:Blurred vision, taste alteration, anosmia, tinnitus, conjunctivitis, dry eyes, tearing.
Urogenital:Flank pain, gynecomastia, impotence.
Miscellaneous:A symptom complex has been reported which may include some or all of the following: a positive ANA, an elevated erythrocyte sedimentation rate, arthralgia/arthritis, myalgia/myositis, fever, serositis, vasculitis, leukocytosis, eosinophilia, photosensitivity, dermatologic manifestations.
Related/similar drugs
7. Drug Interactions
7.1 Non-Steroidal Anti-Inflammatory Agents (NSAIDs) Including Selective Cyclooxygenase-2 Inhibitors (COX-2 Inhibitors)
In patients who are elderly, volume-depleted (including those on diuretic therapy), or with compromised renal function, co-administration of NSAIDs, including selective COX-2 inhibitors, with ACE inhibitors, including enalapril, may result in deterioration of renal function, including possible acute renal failure. These effects are usually reversible. Monitor renal function periodically in patients receiving enalapril and NSAID therapy.
In a clinical pharmacology study, indomethacin or sulindac was administered to hypertensive patients receiving enalapril maleate. In this study, there was no evidence of a blunting of the antihypertensive action of enalapril maleate. However, reports suggest that NSAIDs may diminish the antihypertensive effect of ACE inhibitors.
7.2 Dual Blockade of the Renin-Angiotensin System (RAS)
Dual blockade of the RAS with angiotensin receptor blockers, ACE inhibitors, or aliskiren is associated with increased risks of hypotension, hyperkalemia, and changes in renal function (including acute renal failure) compared to monotherapy. In most patients no benefit has been associated with using two RAS inhibitors concomitantly. In general, avoid combined use of RAS inhibitors. Closely monitor blood pressure, renal function and electrolytes in patients on enalapril and other agents that affect the RAS.
Do not co-administer aliskiren with enalapril in patients with diabetes. Avoid use of aliskiren with enalapril in patients with renal impairment (GFR <60 mL/min).
7.3 Agents Increasing Serum Potassium
Enalapril attenuates potassium loss caused by thiazide-type diuretics. Potassium-sparing diuretics (e.g., spironolactone, triamterene, or amiloride), potassium supplements, or potassium-containing salt substitutes may lead to significant increases in serum potassium.
7.4 Lithium
Lithium toxicity has been reported in patients receiving enalapril and lithium concomitantly which was generally reversible. It is recommended that serum lithium levels be monitored frequently if enalapril is administered concomitantly with lithium.
7.5 Gold
Nitritoid reactions (symptoms include facial flushing, nausea, vomiting, and hypotension) have been reported rarely in patients on therapy with injectable gold (sodium aurothiomalate) and concomitant ACE inhibitor therapy including enalapril.
7.6 mTOR Inhibitors
Patients taking concomitant mTOR inhibitor (e.g., temsirolimus, sirolimus, everolimus) therapy may be at increased risk for angioedema [see Warnings and Precautions ( 5.2)] .
7.7 Neprilysin Inhibitor
Patients taking concomitant neprilysin inhibitors may be at increased risk for angioedema [see Warnings and Precautions ( 5.2)] .
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
Enalapril can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Most epidemiologic studies examining fetal abnormalities after exposure to antihypertensive use in the first trimester have not distinguished drugs affecting the renin-angiotensin system from other antihypertensive agents. When pregnancy is detected, discontinue enalapril as soon as possible. The estimated background risk of major birth defects and miscarriage for the indicated population(s) are unknown. In the general U.S. population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Adverse reactions in the fetus or in neonates with a history of in utero exposure to enalapril maleate.
Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy can result in the following: reduced fetal renal function leading to anuria and renal failure, oligohydramnios, fetal lung hypoplasia, skeletal deformations, including skull hypoplasia, hypotension, and death. In the unusual case that there is no appropriate alternative to therapy with drugs affecting the renin-angiotensin system for a particular patient, apprise the mother of the potential risk to the fetus.
Perform serial ultrasound examinations to assess the intra-amniotic environment. Fetal testing may be appropriate, based on the week of pregnancy. Patients and physicians should be aware, however, that oligohydraminos may not appear until after the fetus has sustained irreversible injury. Closely observe infants with histories of in uteroexposure to enalapril for hypotension, oliguria, and hyperkalemia. If oliguria or hypotension occurs in neonates with a history of in uteroexposure to enalapril, support blood pressure and renal perfusion. Exchange transfusions or dialysis may be required as a means of reversing hypotension and substituting for disordered renal function.
8.2 Lactation
Risk Summary
Enalapril and enalaprilat have been detected in human breast milk. Because of the potential for severe adverse reactions in the breastfed infant, including hypotension, hyperkalemia, and renal impairment, advise women not to breastfeed during treatment with enalapril.
8.4 Pediatric Use
Neonates with a history of in utero exposure to enalapril maleate
If oliguria or hypotension occurs, direct attention toward support of blood pressure and renal perfusion. Exchange transfusions or dialysis may be required as a means of reversing hypotension and/or substituting for disordered renal function. Enalapril, which crosses the placenta, has been removed from neonatal circulation by peritoneal dialysis with some clinical benefit, and theoretically may be removed by exchange transfusion, although there is no experience with the latter procedure.
Pediatric patients with heart failure or asymptomatic left ventricular dysfunction
Safety and effectiveness of enalapril have not been established in pediatric patients with heart failure or asymptomatic left ventricular dysfunction.
8.5 Geriatric Use
This drug is known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
8.6 Race
ACE inhibitors, including enalapril, as monotherapy have an effect on blood pressure that is less in black patients than in non-blacks.
8.7 Renal Impairment
Use a lower initial dose of enalapril in patients undergoing hemodialysis and in patients whose eGFR is ≤ 30 mL/min [see Clinical Pharmacology ( 12.3)] .
10. Overdosage
Limited data are available in regard to overdosage in humans.
Single oral doses of enalapril above 1,000 mg/kg and ≥1,775 mg/kg were associated with lethality in mice and rats, respectively.
The most likely manifestation of overdosage would be hypotension, for which the usual treatment would be intravenous infusion of normal saline solution.
Enalaprilat may be removed from general circulation by hemodialysis and has been removed from neonatal circulation by peritoneal dialysis.
11. Enalapril Oral Solution Description
Enalapril maleate oral solution is the maleate salt of enalapril, the ethyl ester prodrug of a long-acting angiotensin-converting enzyme inhibitor, enalaprilat. Enalapril maleate is chemically described as (S)-1-[N-[1-(ethoxycarbonyl)-3-phenylpropyl]-L-alanyl]-L-proline, (Z)-2-butenedioate salt (1:1). Its empirical formula is C 20H 28N 2O 5•C 4H 4O 4, and its structural formula is:
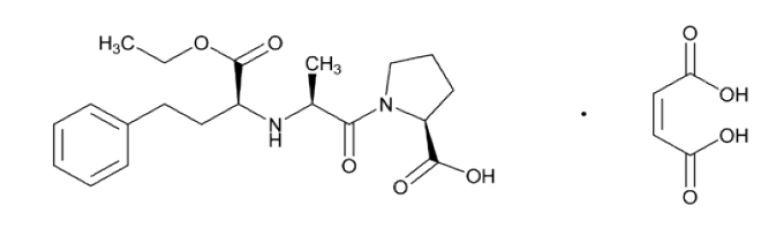
Enalapril maleate, USP is an off-white, crystalline powder with a molecular weight of 492.52. It is practically insoluble in n-heptane (non-polar organic solvent), slightly soluble in acetone (semi-polar organic solvent), sparingly soluble in water, soluble in alcohol, freely soluble in methanol and in dimethyl formamide.
Enalapril maleate oral solution is a ready-to-use oral solution. Each 1 mL contains 1 mg of enalapril maleate, USP equivalent to 0.764 mg of enalapril. Inactive ingredients include methylparaben, mixed berry flavor, propylene glycol, propylparaben, purified water, sorbitol solution 70%, and sucralose. It may also contain hydrochloric acid or sodium hydroxide for pH adjustment. Enalapril maleate oral solution is clear and colorless.
12. Enalapril Oral Solution - Clinical Pharmacology
12.1 Mechanism of Action
Enalapril, after hydrolysis to enalaprilat, inhibits angiotensin-converting enzyme (ACE) in human subjects and animals. ACE is a peptidyl dipeptidase that catalyzes the conversion of angiotensin I to the vasoconstrictor substance, angiotensin II. Angiotensin II also stimulates aldosterone secretion by the adrenal cortex. The beneficial effects of enalapril in heart failure appear to result primarily from suppression of the renin-angiotensin-aldosterone system. Inhibition of ACE results in decreased plasma angiotensin II, which leads to decreased vasopressor activity and to decreased aldosterone secretion. Although the latter decrease is small, it results in small increases of serum potassium. In hypertensive patients treated with enalapril maleate tablets alone for up to 48 weeks, mean increases in serum potassium of approximately 0.2 mEq/L were observed. In patients treated with enalapril maleate tablets plus a thiazide diuretic, there was essentially no change in serum potassium [see Warnings and Precautions ( 5.6)] . Removal of angiotensin II negative feedback on renin secretion leads to increased plasma renin activity.
ACE is identical to kininase, an enzyme that degrades bradykinin. Whether increased levels of bradykinin, a potent vasodepressor peptide, play a role in the therapeutic effects of enalapril remains to be elucidated.
12.2 Pharmacodynamics
Heart Failure
In trials in patients treated with digitalis and diuretics, treatment with enalapril resulted in decreased systemic vascular resistance, blood pressure, pulmonary capillary wedge pressure and heart size, and increased cardiac output and exercise tolerance. Heart rate was unchanged or slightly reduced, and mean ejection fraction was unchanged or increased. There was a beneficial effect on severity of heart failure as measured by the New York Heart Association (NYHA) classification and on symptoms of dyspnea and fatigue. Hemodynamic effects were observed after the first dose, and appeared to be maintained in uncontrolled studies lasting as long as four months. Effects on exercise tolerance, heart size, and severity and symptoms of heart failure were observed in placebo-controlled studies lasting from eight weeks to over one year.
12.3 Pharmacokinetics
The pharmacokinetics of ready-to-use enalapril maleate oral solution was shown to be bioequivalent to that of reconstituted enalapril maleate powder for oral solution under fasted conditions.
Reconstituted enalapril maleate powder for oral solution was shown to be bioequivalent to Vasotec ®tablets. Reconstituted enalapril maleate powder for oral solution was also evaluated under fed and fasted conditions. A high-fat meal reduced the C maxof enalapril and enalaprilat by 46% and 36%, respectively. The exposure, as measured by AUC, to enalaprilat was reduced by 23%. The time to peak concentrations (C max) was delayed by 20 minutes for enalapril and 62 minutes for enalaprilat. The trough plasma concentrations of enalapril (from 6 to 12 hours) and enalaprilat (from 16 to 36 hours) are similar between fasted and fed administrations.
Adults
Following oral administration of enalapril maleate tablets, peak serum concentrations of enalapril occur within about one hour. Based on urinary recovery, the extent of absorption of enalapril is approximately 60%. Enalapril absorption is not influenced by the presence of food in the gastrointestinal tract. Following absorption, enalapril is hydrolyzed to enalaprilat, which is a more potent angiotensin-converting enzyme inhibitor than enalapril; enalaprilat is poorly absorbed when administered orally. Peak serum concentrations of enalaprilat occur three to four hours after an oral dose of enalapril maleate. Excretion of enalapril is primarily renal.
Approximately 94% of the dose is recovered in the urine and feces as enalaprilat or enalapril. The principal components in urine are enalaprilat, accounting for about 40% of the dose, and intact enalapril. There is no evidence of metabolites of enalapril, other than enalaprilat.
The serum concentration profile of enalaprilat exhibits a prolonged terminal phase, apparently representing a small fraction of the administered dose that has been bound to ACE. The amount bound does not increase with dose, indicating a saturable site of binding. The effective half-life for accumulation of enalaprilat following multiple doses of enalapril maleate is 11 hours.
The disposition of enalapril and enalaprilat in patients with renal insufficiency is similar to that in patients with normal renal function until the glomerular filtration rate is 30 mL/min or less. With glomerular filtration rate ≤30 mL/min, peak and trough enalaprilat levels increase, time to peak concentration increases, and time to steady state may be delayed. The effective half-life of enalaprilat following multiple doses of enalapril maleate is prolonged at this level of renal insufficiency. Enalaprilat is dialyzable at the rate of 62 mL/min. Administering enalapril 1 hour after hemodialysis led to a reduction of approximately 50% in the enalaprilat AUC 0-6 hcompared to off dialysis days.
Pediatric Patients
A multiple dose pharmacokinetic study was conducted in 40 hypertensive male and female pediatric patients aged 2 months to ≤16 years following daily oral administration of 0.07 to 0.14 mg/kg enalapril maleate. At steady state, the mean effective half-life for accumulation of enalaprilat was 14 hours and the mean urinary recovery of total enalapril and enalaprilat in 24 hours was 68% of the administered dose. Conversion of enalapril to enalaprilat was in the range of 63 to 76%. The overall results of this study indicate that the pharmacokinetics of enalapril in hypertensive children aged 6 years to ≤16 years are consistent across the studied age groups and consistent with pharmacokinetic historical data in healthy adults. Hypertensive children aged 2 months to 6 years required higher weight-based doses (0.13 mg/kg and 0.11 mg/kg) compared to the older age groups (0.11 mg/kg and 0.07 mg/kg), to achieve similar steady-state AUC.
In the above pediatric study, enalapril maleate was given as tablets and for those children and infants who were unable to swallow tablets or who required a lower dose than is available in tablet form, enalapril was administered in a suspension formulation.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
There was no evidence of a tumorigenic effect when enalapril was administered for 106 weeks to male and female rats at doses up to 90 mg/kg/day or for 94 weeks to male and female mice at doses up to 90 and 180 mg/kg/day, respectively. These doses are 26 times (in rats and female mice) and 13 times (in male mice) the maximum recommended human daily dose (MRHDD) when compared on a body surface area basis.
Neither enalapril maleate nor the active diacid was mutagenic in the Ames microbial mutagen test with or without metabolic activation. Enalapril was also negative in the following genotoxicity studies: rec-assay, reverse mutation assay with E. coli, sister chromatid exchange with cultured mammalian cells, and the micronucleus test with mice, as well as in an in vivocytogenic study using mouse bone marrow.
There were no adverse effects on reproductive performance of male and female rats treated with up to 90 mg/kg/day of enalapril (26 times the MRHDD when compared on a body surface area basis).
13.2 Animal Toxicology and/or Pharmacology
In several experimental published studies, rat pups exposed to daily enalapril from birth to post-natal Day 13 (the period of nephrogenesis in this species) developed irreversible renal toxicity. In contrast, treatment after post-natal Day 14 was not toxic to the more mature kidney. Rat kidney development at birth and at 14 days is similar to the human at mid-trimester and in infancy, respectively. The toxic dosages in these studies were about 10X, on a mg/m 2basis, the highest recommended oral (0.58 mg/kg/day) pediatric dosages to treat hypertension. Lower dosages were not studied.
14. Clinical Studies
14.1 Heart Failure, Mortality Trials
In a multicenter, placebo-controlled clinical trial, 2,569 patients with all degrees of symptomatic heart failure and ejection fraction ≤35 percent were randomized to placebo or enalapril and followed for up to 55 months (SOLVD-Treatment). Use of enalapril was associated with an 11 percent reduction in all-cause mortality and a 30 percent reduction in hospitalization for heart failure. Diseases that excluded patients from enrollment in the study included severe stable angina (>2 attacks/day), hemodynamically significant valvular or outflow tract obstruction, renal failure (creatinine >2.5 mg/dL), cerebral vascular disease (e.g., significant carotid artery disease), advanced pulmonary disease, malignancies, active myocarditis and constrictive pericarditis. The mortality benefit associated with enalapril does not appear to depend upon digitalis being present.
A second multicenter trial used the SOLVD protocol for study of asymptomatic or minimally symptomatic patients. SOLVD-Prevention patients, who had left ventricular ejection fraction ≤35% and no history of symptomatic heart failure, were randomized to placebo (n = 2,117) or enalapril (n = 2,111) and followed for up to 5 years. The majority of patients in the SOLVD-Prevention trial had a history of ischemic heart disease. A history of myocardial infarction was present in 80 percent of patients, current angina pectoris in 34 percent, and a history of hypertension in 37 percent. No statistically significant mortality effect was demonstrated in this population. Enalapril-treated subjects had 32% fewer first hospitalizations for heart failure, and 32% fewer total heart failure hospitalizations. Compared to placebo, 32 percent fewer patients receiving enalapril developed symptoms of overt heart failure. Hospitalizations for cardiovascular reasons were also reduced. There was an insignificant reduction in hospitalizations for any cause in the enalapril treatment group (for enalapril vs. placebo, respectively, 1,166 vs. 1,201 first hospitalizations, 2,649 vs. 2,840 total hospitalizations), although the study was not powered to look for such an effect.
The SOLVD-Prevention trial was not designed to determine whether treatment of asymptomatic patients with low ejection fraction would be superior, with respect to preventing hospitalization, to closer follow-up and use of enalapril at the earliest sign of heart failure. However, under the conditions of follow-up in the SOLVD-Prevention trial (every 4 months at the study clinic; personal physician as needed), 68% of patients on placebo who were hospitalized for heart failure had no prior symptoms recorded which would have signaled initiation of treatment.
The SOLVD-Prevention trial was also not designed to show whether enalapril modified the progression of underlying heart disease.
In another multicenter, placebo-controlled trial (CONSENSUS) limited to patients with NYHA Class IV congestive heart failure and radiographic evidence of cardiomegaly, use of enalapril was associated with improved survival. The results are shown in the following table.
| CONSENSUS Survival Rates | ||
| SURVIVAL (%) | ||
| Six Months | One Year | |
| VASOTEC (n = 127) | 74 | 64 |
| Placebo (n = 126) | 56 | 48 |
In both CONSENSUS and SOLVD-Treatment trials, patients were also usually receiving digitalis, diuretics or both.
16. How is Enalapril Oral Solution supplied
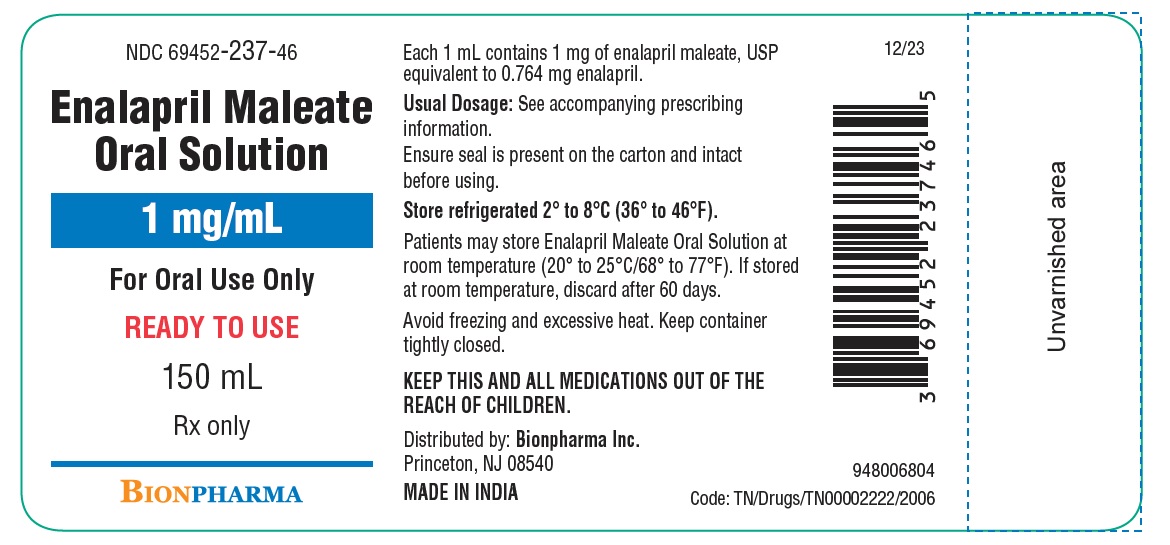
Enalapril maleate oral solution is a ready-to-use solution that contains 1 mg/mL of enalapril maleate, USP. It is a clear, colorless oral solution with a mixed berry flavor, packaged in a 150 mL, white, round, high-density polyethylene bottle with a white, polypropylene, child-resistant cap and placed in a carton with tamper-evident seal. Each bottle contains 150 mL.
NDC 69452-237-46
Store refrigerated (2° to 8°C/36° to 46°F) in a tightly closed container. Protect from freezing and excessive heat. Patients may store enalapril maleate oral solution at room temperature (20° to 25°C/68° to 77°F) for up to 60 days.
17. Patient Counseling Information
- Pregnancy
Tell female patients of childbearing age about the consequences of exposure to enalapril during pregnancy. Discuss treatment options with women planning to become pregnant. Patients should be asked to report pregnancies to their physicians as soon as possible.
- Angioedema
Angioedema, including laryngeal edema, may occur at any time during treatment with angiotensin-converting enzyme inhibitors, including enalapril. Advise patients to report immediately any signs or symptoms suggesting angioedema (swelling of face, extremities, eyes, lips, or tongue, or difficulty in swallowing or breathing) and to consult with the prescribing physician before taking more drug.
- Hypotension
Caution patients to report lightheadedness, especially during the first few days of therapy. If actual syncope occurs, tell patients to discontinue the drug until they have consulted with the prescribing physician.
Tell patients that excessive perspiration and dehydration may lead to an excessive fall in blood pressure because of reduction in fluid volume. Other causes of volume depletion such as vomiting or diarrhea may also lead to a fall in blood pressure; advise patients to consult with their physician.
- Hyperkalemia
Tell patients to consult their physician prior to using salt substitutes containing potassium.
Vasotec is a registered trademark of Valeant International Bermuda.
Distributed by: Bionpharma Inc.
Princeton, NJ 08540
MADE IN INDIA
FDA-06
December 2023
948026816
| ENALAPRIL MALEATE ORAL SOLUTION
enalapril maleate solution |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Bionpharma Inc. (079637826) |
| Registrant - Bionpharma Inc. (079637826) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Novitium Pharma LLC | 080301870 | manufacture(69452-237) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| CoreRx Inc. | 780516717 | manufacture(69452-237) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| OrBion Pharmaceuticals Private Limited | 854403569 | manufacture(69452-237) | |
Frequently asked questions
More about enalapril
- Check interactions
- Compare alternatives
- Reviews (32)
- Drug images
- Side effects
- Dosage information
- Patient tips
- During pregnancy
- Drug class: Angiotensin Converting Enzyme Inhibitors
- Breastfeeding
- En español