Coreg CR: Package Insert / Prescribing Info
Package insert / product label
Generic name: carvedilol phosphate
Dosage form: capsule, extended release
Drug class: Non-cardioselective beta blockers
Medically reviewed by Drugs.com. Last updated on Apr 29, 2025.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Drug Interactions
- Use In Specific Populations
- Overdosage
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
COREG CR (carvedilol phosphate) extended-release capsules for oral use
Initial U.S. Approval: 1995
Indications and Usage for Coreg CR
Coreg CR Dosage and Administration
Take with food. Do not crush or chew capsules. Individualize dosage and monitor during up-titration. (2)
- Heart failure: Start at 10 mg once daily and increase to 20, 40, and then 80 mg once daily over intervals of at least 2 weeks. Maintain lower doses if higher doses are not tolerated. (2.1)
- Left ventricular dysfunction following myocardial infarction.. Start at 20 mg once daily and increase to 40 mg then 80 mg once daily after intervals of 3 to 10 days. A lower starting dose or slower titration may be used. (2.2)
- Hypertension: Start at 20 mg once daily and increase if needed for blood pressure control to 40 mg then 80 mg once daily over intervals of 1 to 2 weeks. (2.3)
- Elderly patients (>65 years of age): When switching from higher doses of immediate-release carvedilol to COREG CR, a lower starting dose should be considered to reduce the risk of hypotension and syncope. (2.5)
Dosage Forms and Strengths
Capsules: 10 mg, 20 mg, 40 mg, 80 mg (3)
Contraindications
- Bronchial asthma or related bronchospastic conditions. (4)
- Second- or third-degree AV block. (4)
- Sick sinus syndrome. (4)
- Severe bradycardia (unless permanent pacemaker in place). (4)
- Patients in cardiogenic shock or decompensated heart failure requiring the use of IV inotropic therapy. (4)
- Severe hepatic impairment. (2.4, 4)
- History of serious hypersensitivity reaction (e.g., Stevens-Johnson syndrome, anaphylactic reaction, angioedema) to carvedilol or any of the components of COREG CR. (4)
Warnings and Precautions
- Acute exacerbation of coronary artery disease upon cessation of therapy: Do not abruptly discontinue. (5.1)
- Bradycardia, hypotension, worsening heart failure/fluid retention may occur. Reduce the dose as needed. (5.2, 5.3, 5.4)
- Non-allergic bronchospasm (e.g., chronic bronchitis and emphysema): Avoid β-blockers. (4) However, if deemed necessary, use with caution and at lowest effective dose. (5.5)
- Diabetes: May mask symptoms of hypoglycemia and alter glucose levels; monitor.(5.6)
Adverse Reactions/Side Effects
The safety profile of COREG CR was similar to that observed for immediate-release carvedilol. Most common adverse events seen with immediate-release carvedilol (6.1):
- Heart failure and left ventricular dysfunction following myocardial infarction (≥10%): Dizziness, fatigue, hypotension, diarrhea, hyperglycemia, asthenia, bradycardia, weight increase.
- Hypertension (≥5%): Dizziness.
To report SUSPECTED ADVERSE REACTIONS, contact Waylis Therapeutics at 1-844-200-7910 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- CYP P450 2D6 enzyme inhibitors may increase and rifampin may decrease carvedilol levels. (7.1, 7.5)
- Hypotensive agents (e.g., reserpine, MAO inhibitors, clonidine) may increase the risk of hypotension and/or severe bradycardia. (7.2)
- Cyclosporine or digoxin levels may increase. (7.3, 7.4)
- Both digitalis glycosides and β-blockers slow atrioventricular conduction and decrease heart rate. Concomitant use can increase the risk of bradycardia. (7.4)
- Amiodarone may increase carvedilol levels resulting in further slowing of the heart rate or cardiac conduction. (7.6)
- Verapamil- or diltiazem-type calcium channel blockers may affect ECG and/or blood pressure. (7.7)
- Insulin and oral hypoglycemics action may be enhanced. (7.8)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 4/2025
Full Prescribing Information
1. Indications and Usage for Coreg CR
1.1 Heart Failure
COREG CR is indicated for the treatment of mild-to-severe chronic heart failure of ischemic or cardiomyopathic origin, usually in addition to diuretics, ACE inhibitors, and digitalis, to increase survival and, also, to reduce the risk of hospitalization [see Drug Interactions (7.4), Clinical Studies (14.1)].
1.2 Left Ventricular Dysfunction following Myocardial Infarction
COREG CR is indicated to reduce cardiovascular mortality in clinically stable patients who have survived the acute phase of a myocardial infarction and have a left ventricular ejection fraction of less than or equal to 40% (with or without symptomatic heart failure) [see Clinical Studies (14.2)].
1.3 Hypertension
COREG CR is ındicated for the management of essential hypertension [see Clinical Studies (14.3, 14.4)]. It can be used alone or in combination with other antihypertensive agents, especially thiazide-type diuretics [see Drug Interactions (7.2)].
2. Coreg CR Dosage and Administration
COREG CR is an extended-release capsule intended for once-daily administration. Patients controlled with immediate-release carvedilol tablets alone or in combination with other medications may be switched to COREG CR extended-release capsules based on the total daily doses shown in Table 1.
| Daily Dose of Immediate-Release Carvedilol Tablets | Daily Dose of COREG CR Capsules* |
|---|---|
|
|
| 6.25 mg (3.125 mg twice daily) | 10 mg once daily |
| 12.5 mg (6.25 mg twice daily) | 20 mg once daily |
| 25 mg (12.5 mg twice daily) | 40 mg once daily |
| 50 mg (25 mg twice daily) | 80 mg once daily |
COREG CR should be taken once daily in the morning with food. COREG CR should be swallowed as a whole capsule. COREG CR and/or its contents should not be crushed, chewed, or taken in divided doses.
Alternative Administration
The capsules may be carefully opened and the beads sprinkled over a spoonful of applesauce. The applesauce should not be warm because it could affect the modified-release properties of this formulation. The mixture of drug and applesauce should be consumed immediately in its entirety. The drug and applesauce mixture should not be stored for future use. Absorption of the beads sprinkled on other foods has not been tested.
2.1 Heart Failure
DOSAGE MUST BE INDIVIDUALIZED AND CLOSELY MONITORED BY A PHYSICIAN DURING UP-TITRATION. Prior to initiation of COREG CR, it is recommended that fluid retention be minımized. The recommended starting dose of COREG CR is 10 mg once daily for 2 weeks. Patients who tolerate a dose of 10 mg once daily may have their dose increased to 20, 40, and 80 mg over successive intervals of at least 2 weeks. Patients should be maintained on lower doses if higher doses are not tolerated.
Patients should be advised that initiation of treatment and (to a lesser extent) dosage increases may be associated with transient symptoms of dizziness or lightheadedness (and rarely syncope) within the first hour after dosing. Thus, during these periods, they should avoid situations such as driving or hazardous tasks, where symptoms could result in injury. Vasodilatory symptoms often do not require treatment, but it may be useful to separate the time of dosing of COREG CR from that of the ACE inhibitor or to reduce temporarily the dose of the ACE inhıbitor. The dose of COREG CR should not be increased until symptoms of worsening heart failure or vasodilation have been stabilized.
Fluid retention (with or without transient worsening heart failure symptoms) should be treated by an increase in the dose of diuretics.
The dose of COREG CR should be reduced if patients experience bradycardia (heart rate less than 55 beats per minute).
Episodes of dizziness or fluid retention during initiation of COREG CR can generally be managed without discontinuation of treatment and do not preclude subsequent successful titration of, or a favorable response to, COREG CR.
2.2 Left Ventricular Dysfunction following Myocardial Infarction
DOSAGE MUST BE INDIVIDUALIZED AND MONITORED DURING UP-TITRATION. Treatment with COREG CR may be started as an inpatient or outpatient and should be started after the patient is hemodynamically stable and fluid retention has been minimized. It is recommended that COREG CR be started at 20 mg once daily and increased after 3 to 10 days, based on tolerabılity, to 40 mg once daily, then again to the target dose of 80 mg once daily. A lower starting dose may be used (10 mg once daily) and/or the rate of up-titration may be slowed if clinically indicated (e.g., due to low blood pressure or heart rate, or fluid retention). Patients should be maintained on lower doses if higher doses are not tolerated. The recommended dosing regimen need not be altered in patients who received treatment with an IV or oral β-blocker during the acute phase of the myocardial infarction.
2.3 Hypertension
DOSAGE MUST BE INDIVIDUALIZED. The recommended starting dose of COREG CR is 20 mg once daily. If this dose is tolerated, using standing systolic pressure measured about 1 hour after dosing as a guide, the dose should be maintained for 7 to 14 days, and then increased to 40 mg once daily if needed, based on trough blood pressure, again using standing systolic pressure 1 hour after dosing as a guide for tolerance. This dose should also be maintained for 7 to 14 days and can then be adjusted upward to 80 mg once daily if tolerated and needed.Although not specifically studied, it is anticipated the full antihypertensive effect of COREG CR would be seen within 7 to 14 days as had been demonstrated with immediate-release carvedilol. Total daily dose should not exceed 80 mg.
Concomitant administration with a diuretic can be expected to produce additive effects and exaggerate the orthostatic component of carvedilol action.
2.4 Hepatic Impairment
COREG CR should not be given to patients with severe hepatic ımpairment [see Contraindications (4)].
2.5 Geriatric Use
When switching elderly patients (aged 65 years or older) who are taking the higher doses of immediate-release carvedilol tablets (25 mg twice daily) to COREG CR, a lower starting dose (40 mg) of COREG CR is recommended to minimize the potential for dizziness, syncope, or hypotension [see Dosage and Administration (2)]. Patients who have switched and who tolerate COREG CR should, as appropriate, have their dose increased after an interval of at least 2 weeks [see Use in Specific Populations (8.5)].
3. Dosage Forms and Strengths
The hard gelatin capsules are filled with white to off-white microparticles and are available in the following strengths:
- 10 mg - white and green capsule shell printed with "GSLGK" and "10 mg"
- 20 mg - white and yellow capsule shell printed with "GSMHV" and "20 mg"
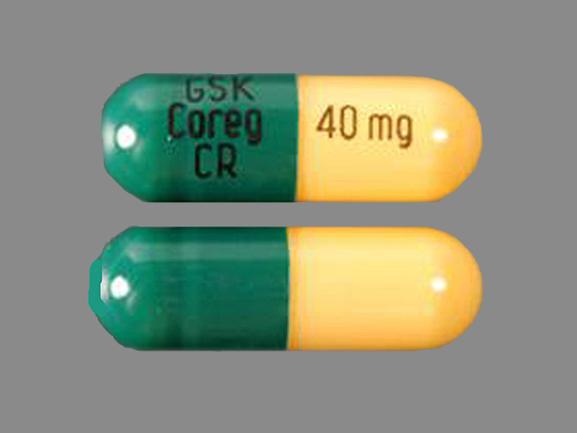
- 40 mg - yellow and green capsule shell printed with "GSETX" and "40 mg"
- 80 mg - white capsule shell printed with "GSF1L" and "80 mg"
4. Contraindications
COREG CR is contraindicated in the following conditions:
- Bronchial asthma or related bronchospastic conditions. Deaths from status asthmaticus have been reported following single doses of immediate-release carvedilol.
- Second- or third-degree AV block.
- Sick sinus syndrome.
- Severe bradycardia (unless a permanent pacemaker is in place).
- Patients with cardiogenic shock or who have decompensated heart failure requiring the use of intravenous inotropic therapy. Such patients should first be weaned from intravenous therapy before initiating COREG CR.
- Patients with severe hepatic impairment.
- Patients with a history of a serious hypersensitivity reaction (e.g., Stevens-Johnson syndrome, anaphylactic reaction, angioedema) to carvedilol or any of the components of COREG CR.
5. Warnings and Precautions
In clinical trials of COREG CR in subjects with hypertension (338 subjects) and in subjects with left ventricular dysfunction following a myocardial infarction or heart failure (187 subjects), the profile of adverse events observed with carvedilol phosphate was generally similar to that observed with the administration of immediate-release carvedilol. Therefore, the information included within this section is based on data from controlled clinical trials with COREG CR as well as immediate-release carvedilol.
5.1 Cessation of Therapy
Patients with coronary artery disease, who are being treated with COREG CR, should be advised against abrupt discontinuation of therapy. Severe exacerbation of angina and the occurrence of myocardial infarction and ventricular arrhythmias have been reported in patients with angina following the abrupt discontinuation of therapy with β-blockers. The last 2 complications may occur with or without preceding exacerbation of the angina pectoris. As with other β-blockers, when discontinuation of COREG CR is planned, the patients should be carefully observed and advised to limit physical activity to a minimum. COREG CR should be discontinued over 1 to 2 weeks whenever possible. If the angina worsens or acute coronary insufficiency develops, it is recommended that COREG CR be promptly reinstituted, at least temporarily. Because coronary artery disease is common and may be unrecognized, it may be prudent not to discontinue therapy with COREG CR abruptly even in patients treated only for hypertension or heart failure.
5.2 Bradycardia
In clinical trials with immediate-release carvedilol, bradycardia was reported in about 2% of hypertensive subjects, 9% of subjects with heart failure, and 6.5% of subjects with myocardial infarction and left ventricular dysfunction. Bradycardia was reported in 0.5% of subjects receiving COREG CR in a trial of subjects with heart failure and subjects with myocardial infarction and left ventricular dysfunction. There were no reports of bradycardia in the clinical trial of COREG CR in hypertension. However, if pulse rate drops below 55 beats per minute, the dosage of COREG CR should be reduced.
5.3 Hypotension
In clinical trials of primarily mild-to-moderate heart failure with immediate-release carvedilol, hypotension and postural hypotension occurred in 9.7% and syncope in 3.4% of subjects receiving carvedilol compared with 3.6% and 2.5% of placebo subjects, respectively. The risk for these events was highest during the first 30 days of dosing, corresponding to the up-titration period and was a cause for discontinuation of therapy in 0.7% of carvedilol subjects, compared with 0.4% of placebo subjects. In a long-term, placebo-controlled trial in severe heart failure (COPERNICUS), hypotension and postural hypotension occurred in 15.1% and syncope in 2.9% of subjects with heart failure receiving carvedilol compared with 8.7% and 2.3% of placebo subjects, respectively. These events were a cause for discontinuation of therapy in 1.1% of carvedilol subjects, compared with 0.8% of placebo subjects.
In a trial comparing subjects with heart failure switched to COREG CR or maintained on immediate-release carvedilol, there was a 2-fold increase in the combined incidence of hypotension, syncope, or dizziness in elderly subjects (older than 65 years) switched from the highest dose of carvedilol (25 mg twice daily) to COREG CR 80 mg once daily [see Dosage and Administration (2), Use in Specific Populations (8.5)].
In the clinical trial of COREG CR in hypertensive subjects, syncope was reported in 0.3% of subjects receivıng COREG CR compared with 0% of subjects receiving placebo. There were no reports of postural hypotension in this trial. Postural hypotension occurred in 1.8% and syncope in 0.1% of hypertensive subjects receiving immediate-release carvedilol, primarily following the initial dose or at the time of dose increase and was a cause for discontinuation of therapy in 1% of subjects.
In the CAPRICORN trial of survivors of an acute myocardial infarction with left ventricular dysfunction, hypotension or postural hypotension occurred in 20.2% of subjects receiving carvedilol compared with 12.6% of placebo subjects. Syncope was reported in 3.9% and 1.9% of subjects, respectively. These events were a cause for discontinuation of therapy in 2.5% of subjects receiving carvedilol, compared with 0.2% of placebo subjects.
Starting with a low dose, administration with food, and gradual up-titration should decrease the likelihood of syncope or excessive hypotension [see Dosage and Administration (2.1, 2.2, 2.3)]. During initiation of therapy, the patient should be cautioned to avoid situations such as driving or hazardous tasks, where injury could result should syncope occur.
5.4 Heart Failure/Fluid Retention
Worsening heart failure or fluid retention may occur during up-titration of carvedilol. If such symptoms occur, diuretics should be increased, and the dose of COREG CR should not be advanced until clinical stability resumes [see Dosage and Administration (2)]. Occasionally it is necessary to lower the dose of COREG CR or temporarily discontinue it. Such episodes do not preclude subsequent successful titration of, or a favorable response to, COREG CR. In a placebo -controlled trial of subjects with severe heart failure, worsening heart failure during the first 3 months was reported to a similar degree with immediate-release carvedilol and with placebo. When treatment was maintained beyond 3 months, worsening heart failure was reported less frequently in subjects treated with carvedilol than with placebo. Worsening heart failure observed during long-term therapy is more likely to be related to the patients' underlying disease than to treatment with carvedilol.
5.5 Non-allergic Bronchospasm
Patients with bronchospastic disease (e.g., chronic bronchitis, emphysema) should, in general, not receive β-blockers. COREG CR may be used with caution, however, in patients who do not respond to, or cannot tolerate, other antihypertensive agents. It is prudent, if COREG CR is used, to use the smallest effective dose, so that inhibition of endogenous or exogenous β-agonists is minimized.
In clinical trials of subjects with heart failure, subjects with bronchospastic disease were enrolled if they did not require oral or inhaled medication to treat their bronchospastic disease. In such patients, it is recommended that COREG CR be used with caution. The dosing recommendations should be followed closely, and the dose should be lowered if any evidence of bronchospasm is observed during up-titration.
5.6 Effects on Blood Sugar
Betablockers may prevent early warning signs of hypoglycemia, such as tachycardia, and increase the risk for severe or prolonged hypoglycemia at any time during treatment, especially in patients with diabetes mellitus or children and patients who are fasting (i.e., surgery, not eating regularly, or are vomiting). If severe hypoglycemia occurs, patients should be instructed to seek emergency treatment.
In patients with heart failure and diabetes, carvedilol therapy may lead to worsening hyperglycemia, which responds to intensification of hypoglycemic therapy. It is recommended that blood glucose be monitored when dosing with COREG CR is initiated, adjusted, or discontinued. Trials designed to examine the effects of carvedilol on glycemic control in patients with diabetes and heart failure have not been conducted.
In a trial designed to examine the effects of immediate-release carvedilol on glycemic control in a population with mild-to-moderate hypertension and well-controlled type 2 diabetes mellitus, carvedilol had no adverse effect on glycemic control, based on HbA1c measurements [see Clinical Studies (14.4)].
5.7 Peripheral Vascular Disease
β-blockers can precipitate or aggravate symptoms of arterial insufficiency in patients with peripheral vascular disease. Caution should be exercised in such individuals.
5.8 Deterioration of Renal Function
Rarely, use of carvedilol in patients with heart failure has resulted in deterioration of renal function. Patients at risk appear to be those with low blood pressure (systolic blood pressure less than 100 mm Hg), ischemic heart disease and diffuse vascular disease, and/or underlying renal insufficiency. Renal function has returned to baseline when carvedilol was stopped. In patients with these risk factors, it is recommended that renal function be monitored during up-titration of COREG CR and the drug discontinued or dosage reduced if worsening of renal function occurs.
5.9 Major Surgery
Chronically administered β-blocking therapy should not be routinely withdrawn prior to major surgery; however, the impaired ability of the heart to respond to reflex adrenergic stimuli may augment the risks of general anesthesia and surgical procedures.
5.10 Thyrotoxicosis
β-adrenergic blockade may mask clinical signs of hyperthyroidism, such as tachycardia. Abrupt withdrawal of β-blockade may be followed by an exacerbation of the symptoms of hyperthyroidism or may precipitate thyroid storm.
5.11 Pheochromocytoma
In patients with pheochromocytoma, an α-blocking agent should be initiated prior to the use of any β-blocking agent. Although carvedilol has both α- and β-blocking pharmacologic activities, there has been no experience with its use in this condition. Therefore, caution should be taken in the administration of carvedilol to patients suspected of having pheochromocytoma.
5.12 Prinzmetal's Variant Angina
Agents with non-selective β-blocking activity may provoke chest pain in patients with Prinzmetal's variant angina. There has been no clinical experience with carvedilol in these patients although the α-blocking activity may prevent such symptoms. However, caution should be taken in the administration of COREG CR to patients suspected of having Prinzmetal's variant angina.
5.13 Risk of Anaphylactic Reaction
While taking β-blockers, patients with a history of severe anaphylactic reaction to a variety of allergens may be more reactive to repeated challenge, either accidental, diagnostic, or therapeutic. Such patients may be unresponsive to the usual doses of epinephrine used to treat allergic reaction.
5.14 Intraoperative Floppy Iris Syndrome
Intraoperative Floppy Iris Syndrome (IFIS) has been observed during cataract surgery in some patients treated with alpha-1 blockers (COREG CR is an alpha/beta blocker). This variant of small pupil syndrome is characterized by the combination of a flaccid iris that billows in response to intraoperative irrigation currents, progressive intraoperative miosis despite preoperative dilation with standard mydriatic drugs, and potential prolapse of the iris toward the phacoemulsification incisions. The patient's ophthalmologist should be prepared for possible modifications to the surgical technique, such as utilization of iris hooks, iris dilator rings, or viscoelastic substances. There does not appear to be a benefit of stopping alpha-1 blocker therapy prior to cataract surgery.
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Carvedilol has been evaluated for safety in subjects with heart failure (mild, moderate, and severe), in subjects with left ventricular dysfunction following myocardial infarction, and in hypertensive subjects. The observed adverse event profile was consistent with the pharmacology of the drug and the health status of the subjects in the clinical trials. Adverse events reported for each of these populations reflecting the use of either COREG CR or immediate-release carvedilol are provided below. Excluded are adverse events considered too general to be informative, and those not reasonably associated with the use of the drug because they were associated with the condition being treated or are very common in the treated population. Rates of adverse events were generally similar across demographic subsets (men and women, elderly and non-elderly, blacks and non-blacks). COREG CR has been evaluated for safety in a 4-week (2 weeks of immediate-release carvedilol and 2 weeks of COREG CR) clinical trial (n = 187) which included 157 subjects with stable mild, moderate, or severe chronic heart failure and 30 subjects with left ventricular dysfunction following acute myocardial infarction.The profile of adverse events observed with COREG CR in this small, short-term trial was generally similar to that observed with immediate-release carvedilol. Differences in safety would not be expected based on the similarity in plasma levels for COREG CR and immediate-release carvedilol.
Heart Failure
The following information describes the safety experience in heart failure with immediate-release carvedilol.
Carvedilol has been evaluated for safety in heart failure in more than 4,500 subjects worldwide of whom more than 2,100 participated in placebo-controlled clinical trials. Approximately 60% of the total treated population in placebo-controlled clinıcal trials received carvedilol for at least 6 months and 30% received carvedilol for at least 12 months. In the COMET trial, 1,511 subjects with mild-to-moderate heart failure were treated with carvedilol for up to 5.9 years (mean: 4.8 years). Both in U.S. clinical trials in mild-to-moderate heart failure that compared carvedilol in daily doses up to 100 mg (n = 765) with placebo (n = 437), and in a multinational clinical trial in severe heart failure (COPERNICUS) that compared carvedilol in daily doses up to 50 mg (n = 1,156) with placebo (n = 1,133), discontinuation rates for adverse experiences were similar in carvedilol and placebo subjects. In placebo-controlled clinical trials, the only cause of discontinuation greater than 1% and occurring more often on carvedilol was dizziness (1.3% on carvedilol, 0.6% on placebo in the COPERNICUS trial).
Table 2 shows adverse events reported in subjects with mild-to-moderate heart failure enrolled in U.S. placebo-controlled clinical trials, and with severe heart failure enrolled in the COPERNICUS trial. Shown are adverse events that occurred more frequently in drug-treated subjects than placebo-treated subjects with an incidence of greater than 3% in subjects treated with carvedilol regardless of causality. Median trial medication exposure was 6.3 months for both carvedilol and placebo subjects in the trials of mild-to-moderate heart failure and 10.4 months in the trial of subjects with severe heart failure. The adverse event profile of carvedilol observed in the long-term COMET trial was generally similar to that observed in the U.S. Heart Failure Trials.
| Body System/Adverse Event | Mild-to-Moderate HF | Severe HF | ||
|---|---|---|---|---|
| Carvedilol | Placebo | Carvedilol | Placebo | |
| (n = 765) | (n = 437) | (n = 1,156) | (n = 1,133) | |
| Body as a Whole | ||||
| Asthenia | 7 | 7 | 11 | 9 |
| Fatigue | 24 | 22 | — | — |
| Digoxin level increased | 5 | 4 | 2 | 1 |
| Edema generalized | 5 | 3 | 6 | 5 |
| Edema dependent | 4 | 2 | — | — |
| Cardiovascular | ||||
| Bradycardia | 9 | 1 | 10 | 3 |
| Hypotension | 9 | 3 | 14 | 8 |
| Syncope | 3 | 3 | 8 | 5 |
| Angina pectoris | 2 | 3 | 6 | 4 |
| Central Nervous System | ||||
| Dizziness | 32 | 19 | 24 | 17 |
| Headache | 8 | 7 | 5 | 3 |
| Gastrointestinal | ||||
| Diarrhea | 12 | 6 | 5 | 3 |
| Nausea | 9 | 5 | 4 | 3 |
| Vomiting | 6 | 4 | 1 | 2 |
| Metabolic | ||||
| Hyperglycemia | 12 | 8 | 5 | 3 |
| Weight increase | 10 | 7 | 12 | 11 |
| BUN increased | 6 | 5 | — | — |
| NPN increased | 6 | 5 | — | — |
| Hypercholesterolemia | 4 | 3 | 1 | 1 |
| Edema peripheral | 2 | 1 | 7 | 6 |
| Musculoskeletal | ||||
| Arthralgia | 6 | 5 | 1 | 1 |
| Respiratory | ||||
| Cough increased | 8 | 9 | 5 | 4 |
| Rales | 4 | 4 | 4 | 2 |
| Vision | ||||
| Vision abnormal | 5 | 2 | — | — |
Cardiac failure and dyspnea were also reported in these trials, but the rates were equal or greater in subjects who received placebo.
The following adverse events were reported with a frequency of greater than 1% but less than or equal to 3% and more frequently with carvedilol in either the U.S. placebo-controlled trials in subjects with mild-to-moderate heart failure or in subjects with severe heart failure in the COPERNICUS trial.
Incidence greater than 1% to less than or equal to 3%
Body as a Whole: Allergy, malaise, hypovolemia, fever, leg edema.
Cardiovascular: Fluid overload, postural hypotension, aggravated angina pectoris, AV block, palpitation, hypertension.
Central and Peripheral Nervous System: Hypesthesia, vertigo, paresthesia.
Gastrointestinal: Melena, periodontitis.
Liver and Biliary System: SGPT increased, SGOT increased.
Metabolic and Nutritional: Hyperuricemia, hypoglycemia, hyponatremia, increased alkaline phosphatase, glycosuria, hypervolemia, diabetes mellitus, GGT increased, weight loss, hyperkalemia, creatinine increased.
Musculoskeletal: Muscle cramps.
Platelet, Bleeding, and Clotting: Prothrombin decreased, purpura, thrombocytopenia.
Psychiatric: Somnolence.
Reproductive, male: Impotence.
Special Senses: Blurred vision.
Urinary System: Renal insufficiency, albuminuria, hematuria.
Left Ventricular Dysfunction following Myocardial Infarction
The following information describes the safety experience in left ventricular dysfunction following acute myocardial infarction with immediate-release carvedilol.
Carvedilol has been evaluated for safety in survivors of an acute myocardial infarction with left ventricular dysfunction in the CAPRICORN trial which involved 969 subjects who received carvedilol and 980 who received placebo. Approximately 75% of the subjects received carvedilol for at least 6 months and 53% received carvedilol for at least 12 months. Subjects were treated for an average of 12.9 months and 12.8 months with carvedilol and placebo, respectively.
The most common adverse events reported with carvedilol in the CAPRICORN trial were consistent with the profile of the drug in the U.S. heart failure trials and the COPERNICUS trial. The only additional adverse events reported in CAPRICORN in greater than 3% of the subjects and more commonly on carvedilol were dyspnea, anemia, and lung edema. The following adverse events were reported with a frequency of > 1% but ≤ 3% and more frequently with carvedilol: flu syndrome, cerebrovascular accident, peripheral vascular disorder, hypotonia, depression, gastrointestinal pain, arthritis, and gout. The overall rates of discontinuations due to adverse events were similar in both groups of subjects. In this database, the only cause of discontinuation > 1% and occurring more often on carvedilol was hypotension (1.5% on carvedilol, 0.2% on placebo).
Hypertension
COREG CR was evaluated for safety in an 8-week double-blind trial in 337 subjects with essential hypertension. The profile of adverse events observed with COREG CR was generally simılar to that observed with immediate-release carvedilol. The overall rates of discontinuations due to adverse events were similar between COREG CR and placebo.
| Adverse Event | COREG CR (n = 253) | Placebo (n = 84) |
|---|---|---|
| Nasopharyngitis | 4 | 0 |
| Dizziness | 2 | 1 |
| Nausea | 2 | 0 |
| Edema peripheral | 2 | 1 |
| Nasal congestion | 1 | 0 |
| Paresthesia | 1 | 0 |
| Sinus congestion | 1 | 0 |
| Diarrhea | 1 | 0 |
| Insomnia | 1 | 0 |
The following information describes the safety experience in hypertension with immediate-release carvedilol.
Carvedilol has been evaluated for safety in hypertension in more than 2,193 subjects in U.S. clinical trials and in 2,976 subjects in international clinical trials. Approximately 36% of the total treated population received carvedilol for at least 6 months. In general, carvedilol was well tolerated at doses up to 50 mg daily. Most adverse events reported during carvedilol therapy were of mild to moderate severity. In U.S. controlled clinical trials directly comparing carvedilol monotherapy in doses up to 50 mg (n = 1,142) with placebo (n = 462), 4.9% of carvedilol subjects discontinued for adverse events versus 5.2% of placebo subjects. Although there was no overall difference in discontinuation rates, discontinuations were more common in the carvedilol group for postural hypotension (1% versus 0). The overall incidence of adverse events in U.S. placebo-controlled trials was found to increase with increasing dose of carvedilol. For individual adverse events this could only be distinguished for dizziness, which increased in frequency from 2% to 5% as total daily dose increased from 6.25 mg to 50 mg as single or divıded doses.
Table 4 shows adverse events in U.S. placebo-controlled clinical trials for hypertension that occurred with an incidence of greater than or equal to 1% regardless of causality and that were more frequent in drug-treated subjects than placebo-treated subjects.
| Adverse Event | Carvedilol (n = 1,142) | Placebo (n = 462) |
|---|---|---|
|
||
| Cardiovascular | ||
| Bradycardia | 2 | — |
| Postural hypotension | 2 | — |
| Peripheral edema | 1 | — |
| Central Nervous System | ||
| Dizziness | 6 | 5 |
| Insomnia | 2 | 1 |
| Gastrointestinal | ||
| Diarrhea | 2 | 1 |
| Hematologic | ||
| Thrombocytopenia | 1 | — |
| Metabolic | ||
| Hypertriglyceridemia | 1 | — |
Dyspnea and fatigue were also reported in these trials, but the rates were equal or greater in subjects who received placebo.
The following adverse events not described above were reported as possibly or probably related to carvedilol in worldwide open or controlled trials with carvedilol in subjects with hypertension or heart failure.
Incidence greater than 0.1% to less than or equal to 1%
Cardiovascular: Peripheral ischemia, tachycardia.
Central and Peripheral Nervous System: Hypokinesia.
Gastrointestinal: Bilirubinemia, increased hepatic enzymes (0.2% of hypertension patients and 0.4% of heart failure patients were discontinued from therapy because of increases in hepatic enzymes) [see Adverse Reactions (6.2)].
Psychiatric: Nervousness, sleep disorder, aggravated depression, impaired concentration, abnormal thinking, paranoia, emotional lability.
Respiratory System: Asthma [see Contraindications (4)].
Reproductive, male: Decreased libido.
Skin and Appendages: Pruritus, rash erythematous, rash maculopapular, rash psoriaform, photosensitivity reaction.
Special Senses: Tinnitus.
Urinary System: Micturition frequency increased.
Autonomic Nervous System: Dry mouth, sweating increased.
Metabolic and Nutritional: Hypokalemia, hypertriglyceridemia.
Hematologic: Anemia, leukopenia.
The following events were reported in less than or equal to 0.1% of subjects and are potentially important: complete AV block, bundle branch block, myocardial ischemia, cerebrovascular disorder, convulsions, migraine, neuralgia, paresis, anaphylactoid reaction, alopecia, exfoliative dermatitis, amnesia, GI hemorrhage, bronchospasm, pulmonary edema, decreased hearing, respiratory alkalosis, increased BUN, decreased HDL, pancytopenia, and atypical lymphocytes.
Laboratory Abnormalities
Reversible elevations in serum transaminases (ALT or AST) have been observed during treatment with carvedilol. Rates of transaminase elevations (2- to 3- times the upper limit of normal) observed during controlled clinical trials have generally been similar between subjects treated with carvedilol and those treated with placebo. However, transaminase elevations, confirmed by rechallenge, have been observed with carvedilol. In a long-term, placebo-controlled trial in severe heart failure, subjects treated with carvedilol had lower values for hepatic transaminases than subjects treated with placebo, possibly because carvedilol-induced improvements in cardiac function led to less hepatic congestion and/or improved hepatic blood flow.
Carvedilol therapy has not been associated with clinically significant changes in serum potassium, total triglycerides, total cholesterol, HDL cholesterol, uric acid, blood urea nitrogen, or creatinine. No clinically relevant changes were noted in fasting serum glucose in hypertensive subjects; fasting serum glucose was not evaluated in the heart failure clinical trials.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of COREG or COREG CR. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Related/similar drugs
7. Drug Interactions
7.1 CYP2D6 Inhibitors and Poor Metabolizers
Interactions of carvedilol with potent inhibitors of CYP2D6 isoenzyme (such as quinidine, fluoxetine, paroxetine, and propafenone) have not been studied, but these drugs would be expected to increase blood levels of the R(+) enantiomer of carvedilol [see Clinical Pharmacology (12.3)]. Retrospective analysis of side effects in clinical trials showed that poor 2D6 metabolizers had a higher rate of dizziness during up-titration, presumably resulting from vasodilating effects of the higher concentrations of the α-blocking R(+) enantiomer.
7.2 Hypotensive Agents
Patients taking a β-blocker and a drug that can deplete catecholamines (e.g., reserpine and monoamine oxidase inhibitors) should be observed closely for signs of hypotension and/or severe bradycardia.
Concomitant administration of clonidine with a β-blocker may cause hypotension and bradycardia. When concomitant treatment with a β-blocker and clonidine is to be terminated, the β-blocker should be discontinued first. Clonidine therapy can then be discontinued several days later by gradually decreasing the dosage.
7.3 Cyclosporine
Modest increases in mean trough cyclosporine concentrations were observed following initiation of carvedilol treatment in 21 renal transplant subjects suffering from chronic vascular rejection. In about 30% of subjects, the dose of cyclosporine had to be reduced in order to maintain cyclosporine concentrations within the therapeutic range, while in the remainder no adjustment was needed. On the average for the group, the dose of cyclosporine was reduced about 20% in these subjects. Due to wide interindividual variability in the dose adjustment required, it is recommended that cyclosporine concentrations be monitored closely after initiation of carvedilol therapy and that the dose of cyclosporine be adjusted as appropriate.
7.4 Digitalis Glycosides
Both digitalis glycosides and β-blockers slow atrioventricular conduction and decrease heart rate. Concomitant use can increase the risk of bradycardia. Digoxin concentrations are increased by about 15% when digoxin and carvedilol are administered concomitantly. Therefore, increased monitoring of digoxin is recommended when initiating, adjusting, or discontinuing COREG CR [see Drug-Drug Interactions (12.5)].
7.5 Inducers/Inhibitors of Hepatic Metabolism
Rifampin reduced plasma concentrations of carvedilol by about 70% [see Drug-Drug Interactions (12.5)]. Cimetidine increased area under the curve (AUC) by about 30% but caused no change in Cmax [see Drug-Drug Interactions (12.5)].
7.6 Amiodarone
Amiodarone and its metabolite desethyl amiodarone, inhibitors of CYP2C9, and P-glycoprotein increased concentrations of the S(-) enantiomer of carvedilol by at least 2-fold [see Drug-Drug Interactions (12.5)]. The concomitant administration of amiodarone or other CYP2C9 inhibitors such as fluconazole with COREG CR may enhance the β-blocking activity, resulting in further slowing of the heart rate or cardiac conduction. Patients should be observed for signs of bradycardia or heart block, particularly when one agent is added to pre-existing treatment with the other.
7.7 Calcium Channel Blockers
Conduction disturbance (rarely with hemodynamic compromise) has been observed when COREG CR is coadministered with diltiazem. As with other β-blockers, if COREG CR is administered with calcium channel blockers of the verapamil or diltiazem type, it is recommended that ECG and blood pressure be monitored.
7.8 Insulin or Oral Hypoglycemics
β-blockers properties may enhance the blood-sugar-reducing effect of insulin and oral hypoglycemics. Therefore, in patients taking insulin or oral hypoglycemics, regular monitoring of blood glucose is recommended [see Warnings and Precautions (5.6)].
7.9 Proton Pump Inhibitors
There is no clinically meaningful increase in AUC and Cmax with concomitant administration of carvedilol extended-release capsules with pantoprazole.
7.10 Anesthesia
If treatment with COREG CR is to be continued perioperatively, particular care should be taken when anesthetic agents that depress myocardial function, such as ether, cyclopropane, and trichloroethylene, are used [see Overdosage (10)].
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
Available data regarding use of COREG CR in pregnant women are insufficient to determine whether there are drug-associated risks of adverse developmental outcomes. There are risks to the mother and fetus associated with poorly controlled hypertension in pregnancy. The use of beta blockers during the third trimester of pregnancy may increase the risk of hypotension, bradycardia, hypoglycemia, and respiratory depression in the neonate (see Clinical Considerations). In animal reproduction studies, there was no evidence of adverse developmental outcomes at clinically relevant doses (see Data). Oral administration of carvedilol to pregnant rats during organogenesis resulted in post-implantation loss, decreased fetal body weight, and an increased frequency of delayed fetal skeletal development at maternally toxic doses that were 50 times the maximum recommended human dose (MRHD). In addition, oral administration of carvedilol to pregnant rabbits during organogenesis resulted in increased post-implantation loss at doses 25 times the MRHD (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated populations are unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk: Hypertension in pregnancy increases the maternal risk for preeclampsia, gestational diabetes, premature delivery, and delivery complications (e.g., need for cesarean section and post-partum hemorrhage). Hypertension increases the fetal risk for intrauterine growth restriction and intrauterine death. Pregnant women with hypertension should be carefully monitored and managed accordingly.
Fetal/Neonatal Adverse Reactions: Neonates of women with hypertension who are treated with beta-blockers during the third trimester of pregnancy may be at increased risk for hypotension, bradycardia, hypoglycemia, and respiratory depression. Observe newborns for symptoms of hypotension, bradycardia, hypoglycemia, and respiratory depression and manage accordingly.
Data
Animal Data: Studies performed in rats and rabbits given carvedilol during fetal organogenesis revealed increased post-implantation loss in rats at a maternally toxic dose of 300 mg per kg per day (50 times the MRHD as mg per m2) and in rabbits (in the absence of maternal toxicity) at doses of 75 mg per kg per day (25 times the MRHD as mg per m2). In the rats, there was also a decrease in fetal body weight at 300 mg per kg per day (50 times the MRHD as mg per m2) accompanied by an increased incidence of fetuses with delayed skeletal development. In rats, the no-effect level for embryo-fetal toxicity was 60 mg per kg per day (10 times the MRHD as mg per m2); in rabbits, it was 15 mg per kg per day (5 times the MRHD as mg per m2). In a pre-and post-natal development study in rats administered carvedilol from late gestation through lactation, increased embryo-lethality was observed at a maternally toxic dose of 200 mg per kg per day (approximately 32 times the MRHD as mg per m2), and pup mortality and delays in physical growth/development were observed at 60 mg per kg per day (10 times the MRHD as mg per m2) in the absence of maternal toxicity. The no-effect level was 12 mg per kg per day (2 times the MRHD as mg per m2). Carvedilol was present in fetal rat tissue.
8.2 Lactation
Risk Summary
There are no data on the presence of carvedilol in human milk, the effects on the breastfed infant, or the effects on milk production. Carvedilol is present in the milk of lactating rats. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for COREG CR and any potential adverse effects on the breastfed infant from COREG CR or from the underlying maternal condition.
8.4 Pediatric Use
Effectiveness of carvedilol in patients younger than 18 years has not been established.
In a double-blind trial, 161 children (mean age: 6 years; range: 2 months to 17 years; 45% younger than 2 years) with chronic heart failure [NYHA class II-IV, left ventricular ejection fraction less than 40% for children with a systemic left ventricle (LV), and moderate-severe ventricular dysfunction qualitatively by echo for those with a systemic ventricle that was not an LV] who were receiving standard background treatment were randomized to placebo or to 2 dose levels of carvedilol. These dose levels produced placebo-corrected heart rate reduction of 4 to 6 heart beats per minute, indicative of β-blockade activity. Exposure appeared to be lower in pediatric subjects than adults. After 8 months of follow-up, there was no significant effect of treatment on clinical outcomes. Adverse reactions in this trial that occurred in greater than 10% of subjects treated with immediate-release carvedilol and at twice the rate of placebo-treated subjects included chest pain (17% versus 6%), dizziness (13% versus 2%), and dyspnea (11% versus 0%).
8.5 Geriatric Use
The initial clinical trials of COREG CR in subjects with hypertension, heart failure, and left ventricular dysfunction following myocardial infarction did not include sufficient numbers of subjects aged 65 years or older to determine whether they respond differently from younger patients.
A randomized trial (n = 405) comparing subjects with mild to severe heart failure switched to COREG CR or maintained on immediate-release carvedilol included 220 subjects who were aged 65 years or older. In this elderly subgroup, the combined incidence of dizziness, hypotension, or syncope was 24% (18/75) in subjects switched from the highest dose of immediate-release carvedilol (25 mg twice daily) to the highest dose of COREG CR (80 mg once daily) compared with 11% (4/36) in subjects maintained on immediate-release carvedilol (25 mg twice daily). When switching from the higher doses of immediate-release carvedilol to COREG CR, a lower starting dose is recommended for elderly patients [see Dosage and Administration (2.5)].
The following information is available for trials with immediate-release carvedilol. Of the 765 subjects with heart failure randomized to carvedilol in U.S. clinical trials, 31% (235) were aged 65 years or older, and 7.3% (56) were aged 75 years or older. Of the 1,156 subjects randomized to carvedilol in a long-term, placebo-controlled trial ın severe heart failure, 47% (547) were aged 65 years or older, and 15% (174) were aged 75 years or older. Of 3,025 subjects receiving carvedilol in heart failure trials worldwide, 42% were aged 65 years or older. Of the 975 subjects with myocardial infarction randomized to carvedilol in the CAPRICORN trial, 48% (468) were aged 65 years or older, and 11% (111) were aged 75 years or older. Of the 2,065 hypertensive subjects in U.S. clinical trials of efficacy or safety who were treated with carvedilol, 21% (436) were aged 65 years or older. Of 3,722 subjects receiving immediate-release carvedilol in hypertension clinical trials conducted worldwide, 24% were aged 65 years or older.
With the exception of dizziness in hypertensive subjects (incidence 8.8% in the elderly versus 6% in younger subjects), no overall differences in the safety or effectiveness (see Figures 2 and 4) were observed between the older subjects and younger subjects in each of these populations. Similarly, other reported clinical experience has not identified differences in responses between the elderly and younger subjects, but greater sensitivity of some older individuals cannot be ruled out.
10. Overdosage
Overdosage may cause severe hypotension, bradycardia, cardiac insufficiency, cardiogenic shock, and cardiac arrest. Respiratory problems, bronchospasms, vomiting, lapses of consciousness, and generalized seizures may also occur.
The patient should be placed in a supine position and, where necessary, kept under observation and treated under intensive-care conditions. The following agents may be administered:
For excessive bradycardia: Atropine, 2 mg IV.
To support cardiovascular function: Glucagon, 5 to 10 mg IV rapidly over 30 seconds, followed by a continuous infusion of 5 mg per hour, sympathomimetics (dobutamine, isoprenaline, adrenaline) at doses according to body weight and effect.
If peripheral vasodilation dominates, it may be necessary to administer adrenaline or noradrenaline with continuous monitoring of circulatory conditions. For therapy-resistant bradycardia, pacemaker therapy should be performed. For bronchospasm, β-sympathomimetics (as aerosol or IV) or aminophylline IV should be given. In the event of seizures, slow IV injection of diazepam or clonazepam is recommended.
NOTE: In the event of severe intoxication where there are symptoms of shock, treatment with antidotes must be continued for a sufficiently long period of time consistent with the 7- to 10-hour half-life of carvedilol.
There is no experience of overdosage with COREG CR. Cases of overdosage with carvedilol alone or in combination with other drugs have been reported. Quantities ingested in some cases exceeded 1,000 milligrams. Symptoms experienced included low blood pressure and heart rate. Standard supportive treatment was provided and individuals recovered.
11. Coreg CR Description
Carvedilol phosphate is a nonselective β-adrenergic blocking agent with α1-blocking activity. It is (2RS)-1-(9H-Carbazol-4-yloxy)-3-[[2-(2-methoxyphenoxy)ethyl]amino]propan-2-ol phosphate salt (1:1) hemihydrate. It is a racemic mixture with the following structure:
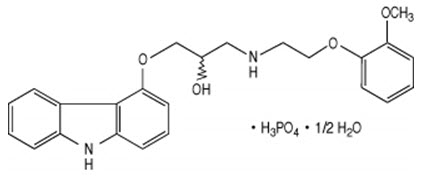
Carvedilol phosphate is a white-to-almost white solid with a molecular weight of 513.5 (406.5 carvedilol free base) and a molecular formula of C24H26N2O4∙H3PO4∙1/2 H2O.
COREG CR is available for once-a-day administration as controlled-release oral capsules contaıning 10, 20, 40, or 80 mg carvedilol phosphate. COREG CR hard gelatin capsules are filled with carvedilol phosphate immediate-release and controlled-release microparticles that are drug-layered and then coated with methacrylic acid copolymers. Inactive ingredients include crospovidone, hydrogenated castor oil, hydrogenated vegetable oil, magnesium stearate, methacrylic acid copolymers, microcrystalline cellulose, and povidone.
12. Coreg CR - Clinical Pharmacology
12.1 Mechanism of Action
Carvedilol is a racemic mixture in which nonselective β-adrenoreceptor blocking activity is present in the S(-) enantiomer and α1-adrenergic blocking activity is present in both R(+) and S(-) enantiomers at equal potency. Carvedilol has no intrinsic sympathomimetic activity.
12.2 Pharmacodynamics
Heart Failure and Left Ventricular Dysfunction following Myocardial Infarction
The basis for the beneficial effects of carvedilol in patients with heart failure and in patients with left ventricular dysfunction following an acute myocardial infarction is not known. The concentration-response relationship for β1-blockade following administration of COREG CR is equivalent (±20%) to immediate-release carvedilol tablets.
Hypertension
The mechanism by which β-blockade produces an antihypertensive effect has not been established.
β-adrenoreceptor blocking activity has been demonstrated in animal and human studies showing that carvedilol (1) reduces cardiac output in normal subjects, (2) reduces exercise- and/or isoproterenol-induced tachycardia, and (3) reduces reflex orthostatic tachycardia. Significant β-adrenoreceptor blocking effect is usually seen within 1 hour of drug administration.
α1-adrenoreceptor blocking activity has been demonstrated in human and animal studies, showing that carvedilol (1) attenuates the pressor effects of phenylephrine, (2) causes vasodilation, and (3) reduces peripheral vascular resistance. These effects contribute to the reduction of blood pressure and usually are seen within 30 minutes of drug administration.
Due to the α1-receptor blocking activity of carvedilol, blood pressure is lowered more in the standing than in the supine position, and symptoms of postural hypotension (1.8%), including rare instances of syncope, can occur. Following oral administration, when postural hypotension has occurred, it has been transient and is uncommon when immediate-release carvedilol is administered with food at the recommended starting dose and titration increments are closely followed [see Dosage and Administration (2)].
In a randomized, double-blind, placebo-controlled trial, the β1-blocking effect of COREG CR, as measured by heart rate response to submaximal bicycle ergometry, was shown to be equivalent to that observed with immediate-release carvedilol at steady state in adult subjects with essential hypertension.
In hypertensive subjects with normal renal function, therapeutic doses of carvedilol decreased renal vascular resistance with no change in glomerular filtration rate or renal plasma flow. Changes in excretion of sodium, potassium, uric acid, and phosphorus in hypertensive patients with normal renal function were similar after carvedilol and placebo.
Carvedilol has little effect on plasma catecholamines, plasma aldosterone, or electrolyte levels, but it does significantly reduce plasma renin activity when given for at least 4 weeks. It also increases levels of atrial natriuretic peptide.
12.3 Pharmacokinetics
Absorption
Carvedilol is rapidly and extensively absorbed following oral administration of immediate-release carvedilol tablets, with an absolute bioavailability of approximately 25% to 35% due to a significant degree of first-pass metabolism. COREG CR extended-release capsules have approximately 85% of the bioavailability of immediate-release carvedilol tablets. For corresponding dosages [see Dosage and Administration (2)], the exposure (AUC, Cmax, trough concentration) of carvedilol as COREG CR extended-release capsules is equivalent to those of immediate-release carvedilol tablets when both are administered with food. The absorption of carvedilol from COREG CR is slower and more prolonged compared with the immediate-release carvedilol tablet with peak concentrations achieved approximately 5 hours after administration. Plasma concentrations of carvedilol increase in a dose-proportional manner over the dosage range of COREG CR 10 to 80 mg. Within-subject and between-subject variabilıty for AUC and Cmax is similar for COREG CR and immediate-release carvedilol.
Effect of Food: Admınistration of COREG CR with a high-fat meal resulted in increases (~20%) in AUC and Cmax compared with COREG CR administered with a standard meal. Decreases in AUC (27%) and Cmax (43%) were observed when COREG CR was administered in the fasted state compared with administration after a standard meal. COREG CR should be taken with food.
In a trial with adult subjects, sprinkling the contents of the COREG CR capsule on applesauce did not appear to have a significant effect on overall exposure (AUC) compared with administration of the intact capsule following a standard meal, but did result in a decrease in Cmax (18%).
Distribution
Carvedilol is more than 98% bound to plasma proteins, primarily with albumin. The plasma-protein binding is independent of concentration over the therapeutic range. Carvedilol is a basic, lipophilic compound with a steady-state volume of distribution of approximately 115 L, indicating substantial distribution into extravascular tissues.
Metabolism and Excretion
Carvedilol is extensively metabolized. Following oral administration of radiolabelled carvedilol to healthy volunteers, carvedilol accounted for only about 7% of the total radioactivity in plasma as measured by AUC. Less than 2% of the dose was excreted unchanged in the urine. Carvedilol is metabolized primarily by aromatic ring oxidation and glucuronidation. The oxidative metabolites are further metabolized by conjugation via glucuronidation and sulfation. The metabolites of carvedilol are excreted primarily via the bile into the feces. Demethylation and hydroxylation at the phenol ring produce 3 active metabolites with β-receptor blocking activity. Based on preclinical studies, the 4'-hydroxyphenyl metabolite is approximately 13 times more potent than carvedilol for β-blockade.
Compared with carvedilol, the 3 active metabolites exhibit weak vasodilating activity. Plasma concentrations of the active metabolites are about one-tenth of those observed for carvedilol and have pharmacokinetics similar to the parent.
Carvedilol undergoes stereoselective first-pass metabolism with plasma levels of R(+)-carvedilol approximately 2 to 3 times higher than S(-)-carvedilol following oral administration of COREG C-R in healthy subjects. Apparent clearance is 90 L per h and 213 L per h for R(+)- and S(-)-carvedilol, respectively.
The primary P450 enzymes responsible for the metabolism of both R(+) and S(-)-carvedilol in human liver microsomes were CYP2D6 and CYP2C9 and to a lesser extent CYP3A4, 2C19, 1A2, and 2E1. CYP2D6 is thought to be the major enzyme in the 4'- and 5'-hydroxylation of carvedilol, with a potential contribution from 3A4. CYP2C9 is thought to be of primary importance in the O-methylation pathway of S(-)-carvedilol.
Carvedilol is subject to the effects of genetic polymorphism with poor metabolizers of debrisoquin (a marker for cytochrome P450 2D6) exhibiting 2- to 3-fold higher plasma concentrations of R(+)-carvedilol compared with extensive metabolizers. In contrast, plasma levels of S(-)-carvedilol are increased only about 20% to 25% in poor metabolizers, indicating this enantiomer is metabolized to a lesser extent by cytochrome P450 2D6 than R(+)-carvedilol. The pharmacokinetics of carvedilol do not appear to be different in poor metabolizers of S-mephenytoin (patients deficient in cytochrome P450 2C19).
12.4 Specific Populations
Heart Failure: Following administration of immediate-release carvedilol tablets, steady-state plasma concentrations of carvedilol and its enantiomers increased proportionally over the dose range in subjects with heart failure. Compared with healthy subjects, subjects with heart failure had increased mean AUC and Cmax values for carvedilol and its enantiomers, with up to 50% to 100% higher values observed in 6 subjects with NYHA class IV heart failure. The mean apparent terminal elimination half-life for carvedilol was similar to that observed in healthy subjects.
For corresponding dose levels [see Dosage and Administration (2)], the steady-state pharmacokinetics of carvedilol (AUC, Cmax, trough concentrations) observed after administration of COREG CR to subjects with chronic heart failure (mild, moderate, and severe) were similar to those observed after administration of immediate-release carvedilol tablets.
Hypertension: For corresponding dose levels [see Dosage and Administration (2)], the pharmacokinetics (AUC, Cmax, and trough concentrations) observed with administration of COREG CR were equivalent (±20%) to those observed with immediate-release carvedilol tablets following repeat dosing in subjects with essential hypertension.
Geriatric: Plasma levels of carvedilol average about 50% higher in the elderly compared with young subjects after administration of immediate-release carvedilol.
Hepatic Impairment: No trials have been performed with COREG CR in subjects with hepatic impairment. Compared with healthy subjects, subjects with severe liver impairment (cirrhosis) exhibit a 4- to 7-fold increase in carvedilol levels. Carvedilol is contraindicated in patients with severe liver impairment.
Renal Impairment: No trials have been performed with COREG CR in subjects with renal impairment. Although carvedilol is metabolized primarily by the liver, plasma concentrations of carvedilol have been reported to be increased in patients with renal impairment after dosing with immediate-release carvedilol. Based on mean AUC data, approximately 40% to 50% higher plasma concentrations of carvedilol were observed in subjects with hypertension and moderate to severe renal impairment compared with a control group of subjects with hypertension and normal renal function. However, the ranges of AUC values were similar for both groups. Changes in mean peak plasma levels were less pronounced, approximately 12% to 26% higher in subjects with impaired renal function.
Consistent with its high degree of plasma protein binding, carvedilol does not appear to be cleared significantly by hemodialysis.
12.5 Drug-Drug Interactions
Since carvedilol undergoes substantial oxidative metabolism, the metabolism and pharmacokinetics of carvedilol may be affected by induction or inhibition of cytochrome P450 enzymes.
The following drug interaction trials were performed with immediate-release carvedilol tablets.
Amiodarone: In a pharmacokinetic trial conducted in 106 Japanese subjects with heart failure, coadministration of small loading and maintenance doses of amiodarone with carvedilol resulted in at least a 2-fold increase in the steady-state trough concentrations of S(-)-carvedilol [see Drug Interactions (7.6)].
Cimetidine: In a pharmacokinetic trial conducted in 10 healthy male subjects, cimetidine (1,000 mg per day) increased the steady-state AUC of carvedilol by 30% with no change in Cmax [see Drug Interactions (7.5)].
Digoxin: Following concomitant administration of carvedilol (25 mg once daily) and digoxin (0.25 mg once daily) for 14 days, steady-state AUC and trough concentrations of digoxin were increased by 14% and 16%, respectively, in 12 subjects with hypertension [see Drug Interactions (7.4)].
Glyburide: In 12 healthy subjects, combined administration of carvedilol (25 mg once daily) and a single dose of glyburide did not result in a clinically relevant pharmacokinetic interaction for either compound.
Hydrochlorothiazide: A single oral dose of carvedilol 25 mg did not alter the pharmacokinetics of a single oral dose of hydrochlorothiazide 25 mg in 12 subjects with hypertension. Likewise, hydrochlorothiazide had no effect on the pharmacokinetics of carvedilol.
Rifampin: In a pharmacokinetic trial conducted in 8 healthy male subjects, rifampin (600 mg daily for 12 days) decreased the AUC and Cmax of carvedilol by about 70% [see Drug Interactions (7.5)].
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In 2-year studies conducted in rats given carvedilol at doses up to 75 mg per kg per day (12 times the MRHD as mg per m2) or in mice given up to 200 mg per kg per day (16 times the MRHD as mg per m2), carvedilol had no carcinogenic effect.
Carvedilol was negative when tested in a battery of genotoxicity assays, including the Ames and the CHO/HGPRT assays for mutagenicity and the in vitro hamster micronucleus and in vivo human lymphocyte cell tests for clastogenicity.
In a combined fertility/developmental/post-natal toxicity study, rats were given carvedilol (12, 60, 300 mg per kg per day) orally by gavage for 2 weeks before mating and through mating, gestation, and weaning for females and for 62 days prior to and through mating for males. At a dosage of 300 mg per kg per day (greater than or equal to 50 times the MRHD as mg per m2) carvedilol was toxic to adult rats (sedation, reduced weight gain) and was associated with a reduced number of successful matings, prolonged mating time, fewer corpora lutea and implants per dam, fewer live pups per litter, and delays in physical growth/development. The no-effect level for overt toxicity and impairment of fertility was 60 mg per kg per day (10 times the MRHD as mg per m2).
14. Clinical Studies
Support for the use of COREG CR extended-release capsules for the treatment of mild-to-severe heart failure and for patients with left ventricular dysfunction following myocardial infarction is based on the equivalence of pharmacokinetic and pharmacodynamic (β1-blockade) parameters between COREG CR and immediate-release carvedilol [see Clinical Pharmacology (12.2, 12.3)].
The clinical trials performed with immediate-release carvedilol in heart failure and left ventricular dysfunction following myocardial infarction are presented below.
14.1 Heart Failure
A total of 6,975 subjects with mild-to-severe heart failure were evaluated in placebo-controlled and active-controlled trials of immediate-release carvedilol.
Mild-to-Moderate Heart Failure
Carvedilol was studied in 5 multicenter, placebo-controlled trials, and in 1 active-controlled trial (COMET trial) involving subjects with mild-to-moderate heart failure.
Four U.S. multicenter, double-blind, placebo-controlled trials enrolled 1,094 subjects (696 randomized to carvedilol) with NYHA class II-III heart failure and ejection fraction less than or equal to 0.35. The vast majority were on digitalis, diuretics, and an ACE inhibitor at trial entry. Subjects were assigned to the trials based upon exercise ability. An Australia-New Zealand double-blind, placebo-controlled trial enrolled 415 subjects (half randomized to immediate-release carvedilol) with less severe heart failure. All protocols excluded subjects expected to undergo cardiac transplantation during the 7.5 to 15 months of double-blind follow-up. All randomized subjects had tolerated a 2-week course on immediate-release carvedilol 6.25 mg twice daily.
In each trial, there was a primary end point, either progression of heart failure (1 U.S. trial) or exercise tolerance (2 U.S. trials meeting enrollment goals and the Australia-New Zealand trial). There were many secondary end points specified in these trials, including NYHA classification, patient and physician global assessments, and cardiovascular hospitalization. Other analyses not prospectively planned included the sum of deaths and total cardiovascular hospitalizations. In situations where the primary end points of a trial do not show a significant benefit of treatment, assignment of significance values to the other results is complex, and such values need to be interpreted cautiously.
The results of the U.S. and Australia-New Zealand trials were as follows:
Slowing Progression of Heart Failure: One U.S. multicenter trial (366 subjects) had as its primary end point the sum of cardiovascular mortality, cardiovascular hospitalization, and sustained increase in heart failure medications. Heart failure progression was reduced, during an average follow-up of 7 months, by 48% (P = 0.008).
In the Australia-New Zealand trial, death and total hospitalizations were reduced by about 25% over 18 to 24 months. In the 3 largest U.S. trials, death and total hospitalizations were reduced by 19%, 39%, and 49%, nominally statistically significant in the last 2 trials. The Australia-New Zealand results were statistically borderline.
Functional Measures: None of the multicenter trials had NYHA classification as a primary end point, but all such trials had it as a secondary end point. There was at least a trend toward improvement in NYHA class in all trials. Exercise tolerance was the primary end point in 3 trials, in none was a statistically significant effect found.
The COMET Trial
In this double-blind trial, 3,029 subjects with NYHA class II-IV heart failure (left ventricular ejection fraction less than or equal to 35%) were randomized to receive either carvedilol (target dose: 25 mg twice daily) or immediate-release metoprolol tartrate (target dose.. 50 mg twice daily). The mean age of the subjects was approximately 62 years, 80% were males, and the mean left ventricular ejection fraction at baseline was 26%. Approximately 96% of the subjects had NYHA class II or III heart failure. Concomitant treatment included diuretics (99%), ACE inhibitors (91%), digitalis (59%), aldosterone antagonists (11%), and "statin" lipid-lowering agents (21%). The mean duration of follow-up was 4.8 years. The mean dose of carvedilol was 42 mg per day.
The trial had 2 primary end points: all-cause mortality and the composite of death plus hospitalization for any reason. The results of COMET are presented in Table 5 below. All-cause mortality carried most of the statistical weight and was the primary determinant of the trial size. All-cause mortality was 34% in the subjects treated with carvedilol and was 40% in the immediate-release metoprolol group (P = 0.0017, hazard ratio = 0.83, 95% CI: 0.74 to 0.93). The effect on mortality was primarily due to a reduction in cardiovascular death. The difference between the 2 groups with respect to the composite end point was not significant (P = 0.122). The estimated mean survival was 8.0 years with carvedilol and 6.6 years with immediate-release metoprolol.
| End Point | Carvedilol n = 1,511 | Metoprolol n = 1,518 | Hazard Ratio | (95% CI) |
|---|---|---|---|---|
| All-cause mortality | 34% | 40% | 0.83 | 0.74 - 0.93 |
| Mortality + all hospitalization | 74% | 76% | 0.94 | 0.86 - 1.02 |
| Cardiovascular death | 30% | 35% | 0.80 | 0.70 - 0.90 |
| Sudden death | 14% | 17% | 0.81 | 0.68 - 0.97 |
| Death due to circulatory failure | 11% | 13% | 0.83 | 0.67 - 1.02 |
| Death due to stroke | 0.9% | 2.5% | 0.33 | 0.18 - 0.62 |
It is not known whether this formulation of metoprolol at any dose or this low dose of metoprolol in any formulation has any effect on survival or hospitalization in patients with heart failure. Thus, this trial extends the time over which carvedilol manifests benefits on survival in heart failure, but it is not evidence that carvedilol improves outcome over the formulation of metoprolol (TOPROL-XL) with benefits in heart failure.
Severe Heart Failure (COPERNICUS)
In a double-blind trial, 2,289 subjects with heart failure at rest or with minimal exertion and left ventricular ejection fraction less than 25% (mean 20%), despite digitalis (66%), diuretics (99%), and ACE inhibitors (89%), were randomized to placebo or carvedilol. Carvedilol was titrated from a starting dose of 3.125 mg twice daily to the maximum tolerated dose or up to 25 mg twice daily over a mınimum of 6 weeks. Most subjects achieved the target dose of 25 mg. The trial was conducted in Eastern and Western Europe, the United States, Israel, and Canada. Similar numbers of subjects per group (about 100) withdrew during the titration period.
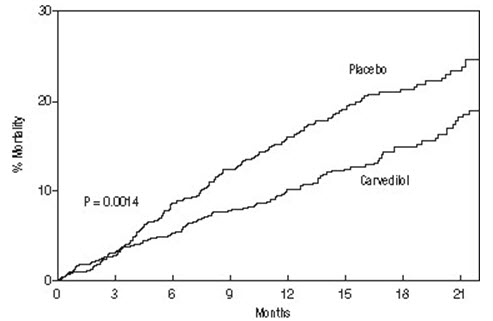
The primary end point of the trial was all-cause mortality, but cause-specific mortality and the risk of death or hospitalization (total, cardiovascular [CV], or heart failure [HF]) were also examined. The developing trial data were followed by a data monitoring committee, and mortality analyses were adjusted for these multiple looks. The trial was stopped after a median follow-up of 10 months because of an observed 35% reduction in mortality (from 19.7% per patient-year on placebo to 12.8% on carvedilol: hazard ratio 0.65, 95% CI: 0.52 to 0.81, P = 0.0014, adjusted) (see Figure 1). The results of COPERNICUS are shown in Table 6.
| End Point | Placebo (n = 1,133) | Carvedilol (n = 1,156) | Hazard Ratio (95% CI) | % Reduction | Nominal P value |
|---|---|---|---|---|---|
| Cardiovascular = CV; Heart failure = HF. | |||||
| Mortality | 190 | 130 | 0.65 (0.52 - 0.81) | 35 | 0.00013 |
| Mortality + all hospitalization | 507 | 425 | 0.76 (0.67 - 0.87) | 24 | 0.00004 |
| Mortality + CV hospitalization | 395 | 314 | 0.73 (0.63 - 0.84) | 27 | 0.00002 |
| Mortality + HF hospitalization | 357 | 271 | 0.69 (0.59 - 0.81) | 31 | 0.000004 |
Figure 1. Survival Analysis for COPERNICUS (Intent-to-Treat)
The effect on mortality was principally the result of a reduction in the rate of sudden death among subjects without worsening heart failure.
Patients' global assessments, in which carvedilol-treated subjects were compared with placebo, were based on pre-specified, periodic patient self-assessments regarding whether clinical status post-treatment showed improvement, worsening, or no change compared with baseline. Subjects treated with carvedilol showed significant improvements in global assessments compared with those treated with placebo in COPERNICUS.
The protocol also specified that hospitalizations would be assessed. Fewer subjects on immediate-release carvedilol than on placebo were hospitalized for any reason (372 versus 432, P=0.0029), for cardiovascular reasons (246 versus 314, P=0.0003), or for worsening heart failure (198 versus 268, P = 0.0001).
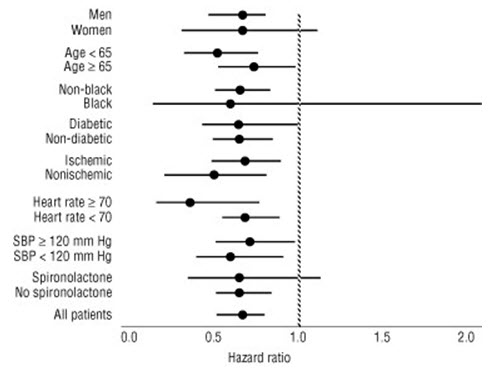
Immediate-release carvedilol had a consistent and beneficial effect on all-cause mortality as well as the combined end points of all-cause mortality plus hospitalization (total, CV, or for heart failure) in the overall trial population and in all subgroups examined, including men and women, elderly and non-elderly, blacks and non-blacks, and diabetics and non-diabetics (see Figure 2).
Figure 2. Effects on Mortality for Subgroups in COPERNICUS
Although the clinical trials used twice-daily dosing, clinical pharmacologic and pharmacokinetic data provide a reasonable basis for concluding that once-daily dosing with COREG CR should be adequate in the treatment of heart failure.
14.2 Left Ventricular Dysfunction following Myocardial Infarction
CAPRICORN was a double-blind trial comparing carvedilol and placebo in 1,959 subjects with a recent myocardial infarction (within 21 days) and left ventricular ejection fraction of less than or equal to 40%, with (47%) or without symptoms of heart failure. Subjects given carvedilol received 6.25 mg twice daily, titrated as tolerated to 25 mg twice daily. Subjects had to have a systolic blood pressure greater than 90 mm Hg, a sitting heart rate greater than 60 beats per minute, and no contraindication to β-blocker use. Treatment of the index infarction included aspirin (85%), IV or oral β-blockers (37%), nitrates (73%), heparin (64%), thrombolytics (40%), and acute angioplasty (12%). Background treatment included ACE inhibitors or angiotensin-receptor blockers (97%), anticoagulants (20%), lipid-lowering agents (23%), and diuretics (34%). Baseline population characteristics included an average age of 63 years, 74% male, 95% Caucasian, mean blood pressure 121/74 mm Hg, 22% with diabetes, and 54% with a history of hypertension. Mean dosage achieved of carvedilol was 20 mg twice daily, mean duration of follow-up was 15 months.
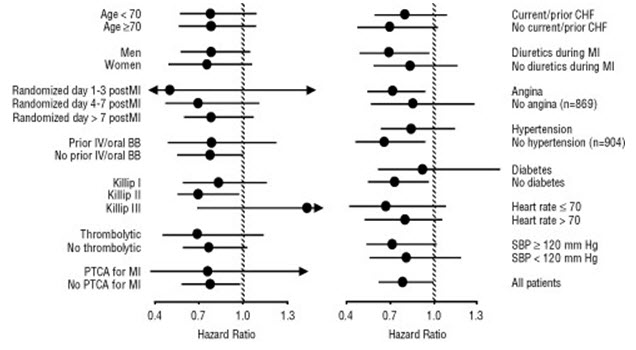
All-cause mortality was 15% in the placebo group and 12% in the carvedilol group, indicating a 23% risk reduction in subjects treated with carvedilol (95% CI: 2% to 40%, P = 0.03), as shown in Figure 3. The effects on mortality in various subgroups are shown in Figure 4. Nearly all deaths were cardiovascular (which were reduced by 25% by carvedilol), and most of these deaths were sudden or related to pump failure (both types of death were reduced by carvedilol). Another trial end point, total mortality and all-cause hospitalization, did not show a significant improvement.
There was also a significant 40% reduction in fatal or non-fatal myocardial infarction observed in the group treated with carvedilol (95% CI: 11% to 60%, P = 0.01). A similar reduction in the risk of myocardial infarction was also observed in a meta-analysis of placebo-controlled trials of carvedilol in heart failure.
Figure 3. Survival Analysis for CAPRICORN (Intent-to-Treat)
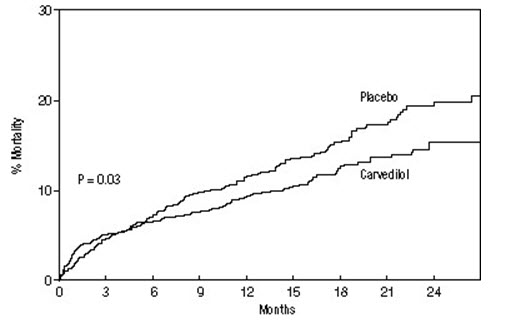
Figure 4. Effects on Mortality for Subgroups in CAPRICORN
Although the clinical trials used twice-daily dosing, clinical pharmacologic and pharmacokinetic data provide a reasonable basis for concluding that once-daily dosing with COREG CR should be adequate in the treatment of left ventricular dysfunction following myocardial infarction.
14.3 Hypertension
A double-blind, randomized, placebo-controlled, 8-week trial evaluated the blood pressure-lowering effects of COREG CR 20 mg, 40 mg, and 80 mg once daily in 338 subjects with essential hypertension (sitting diastolic blood pressure [DBP] greater than or equal to 90 and less than or equal to 109 mm Hg). Of 337 evaluable subjects, a total of 273 subjects (81%) completed the trial. Of the 64 (19%) subjects withdrawn from the trial, 10 (3%) were due to adverse events, 10 (3%) were due to lack of efficacy; the remaining 44 (13%) withdrew for other reasons. The mean age of the subjects was approximately 53 years, 66% were male, and the mean sıtting systolic blood pressure (SBP) and DBP at baseline were 150 mm Hg and 99 mm Hg, respectively. Dose titration occurred at 2-week intervals.
Statistically significant reductions in blood pressure as measured by 24-hour ambulatory blood pressure monitoring (ABPM) were observed with each dose of COREG CR compared with placebo. Placebo-subtracted mean changes from baseline in mean SBP/DBP were -6.1/-4.0 mm Hg, -9.4/-7.6 mm Hg, and 11.8/-9.2 mm Hg for COREG CR 20 mg, 40 mg, and 80 mg, respectively. Placebo-subtracted mean changes from baseline in mean trough (average of hours 20 to 24) SBP/DBP were -3.3/-2.8 mm Hg, 4.9/-5.2 mm Hg , and -8.4/-7.4 mm Hg for COREG CR 20 mg, 40 mg, and 80 mg, respectively. The placebo-corrected trough-to-peak (3 to 7 h) ratio was approximately 0.6 for COREG CR 80 mg. In this trial, assessments of 24-hour ABPM monitoring demonstrated statistically signif cant blood pressure reductions with COREG CR throughout the dosing period (Figure 5).
Figure 5. Changes from Baseline in Systolic Blood Pressure and Diastolic Blood Pressure Measured by 24-Hour ABPM
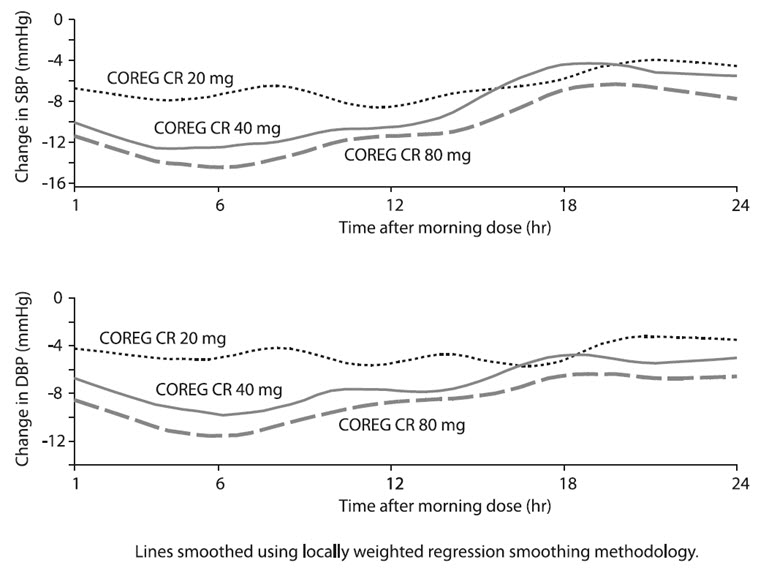
Immediate-release carvedilol was studied in 2 placebo-controlled trials that utilized twice-daily dosing at total daily doses of 12.5 to 50 mg. In these and other trials, the starting dose did not exceed 12.5 mg. At 50 mg per day, COREG reduced sittıng trough (12-hour) blood pressure by about 9/5.5 mm Hg, at 25 mg per day the effect was about 7.5/3.5 mm Hg. Comparisons of trough-to-peak blood pressure showed a trough-to-peak ratio for blood pressure response of about 65%. Heart rate fell by about 7.5 beats per minute at 50 mg per day. In general, as is true for other β-blockers, responses were smaller in black than non-black subjects. There were no age- or gender-related differences in response. The dose-related blood pressure response was accompanied by a dose-related increase in adverse effects [see Adverse Reactions (6)].
14.4 Hypertension with Type 2 Diabetes Mellitus
In a double-blind trial (GEMINI), carvedilol, added to an ACE inhibitor or angiotensin receptor blocker, was evaluated in a population with mild-to-moderate hypertension and well-controlled type 2 diabetes mellitus. The mean HbA1c at baseline was 7.2%. COREG was titrated to a mean dose of 17.5 mg twice daily and maintained for 5 months. COREG had no adverse effect on glycemic control, based on HbA1c measurements (mean change from baseline of 0.02%, 95% CI: -0.06 to 0.10, P = NS) [see Warnings and Precautions (5.6)].
16. How is Coreg CR supplied
The hard gelatin capsules are available in the following strengths:
- 10 mg - white and green capsule shell printed with ' GSLGK" and ' 10 mg"
- 20 mg - white and yellow capsule shell printed with ' GSMHV" and ' 20 mg"
- 40 mg - yellow and green capsule shell printed with "GSETX" and "40 mg"
- 80 mg - white capsule shell printed with ' GSF1L" and ' 80 mg"
- 10 mg bottles of 30: NDC 80725-383-13
- 20 mg bottles of 30: NDC 80725-385-13
- 40 mg bottles of 30: NDC 80725-387-13
- 80 mg bottles of 30: NDC 80725-388-13
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Patients taking COREG CR should be advised of the following:
- Patients should not interrupt or discontinue using COREG CR without a physıcian's advice.
- Patients with heart failure should consult their physician if they experience signs or symptoms of worsening heart failure such as weight gain or increasing shortness of breath.
- Patients may experience a drop in blood pressure when standing, resulting in dizziness and, rarely, fainting. Patients should sit or lie down when these symptoms of lowered blood pressure occur.
- If experiencing dizziness or fatigue, patients should avoid driving or hazardous tasks.
- Patients should consult a physician if they experience dizziness or faintness, in case the dosage should be adjusted.
- Patients should not crush or chew COREG CR.
- Patients should take COREG CR with food.
- Inform patients or caregivers that there is a risk of hypoglycemia when Coreg is given to patients who are fasting or who are vomiting. Instruct patients or caregivers how to monitor for signs of hypoglycemia [see Warnings and Precautions (5.6)].
- Contact lens wearers may experience decreased lacrimation.
COREG CR and COREG are trademarks used under license by Waylis Therapeutics LLC.
The other brand listed is a trademark owned by or licensed to its owner and is not owned by or licensed to Waylis Therapeutics LLC. The maker of this brand is not affiliated with and does not endorse Waylis Therapeutics LLC or its products.
Manufactured for:
Waylis Therapeutics LLC
Rahway, NJ 07065
PATIENT INFORMATION COREG CR (Co-REG) (carvedilol phosphate) Extended-release Capsules
Read the Patient Information that comes with COREG CR before you start taking it and each time you get a refill. There may be new information. This information does not take the place of talking with your doctor about your medical condition or your treatment. If you have any questions about COREG CR, ask your doctor or pharmacist.
What is the most important information I should know about COREG CR?
It is important for you to take your medicine every day as directed by your doctor. If you stop taking COREG CR suddenly, you could have chest pain and a heart attack. If your doctor decides that you should stop taking COREG CR, your doctor may slowly lower your dose over time before stopping it completely.
What is COREG CR?
COREG CR is a prescription medicine that belongs to a group of medicines called ' beta-blockers". COREG CR is used, often with other medicines, for the following conditions:
- to treat patients with certain types of heart failure
- to treat patients who had a heart attack that worsened how well the heart pumps
- to treat patients with high blood pressure (hypertension)
COREG CR is not approved for use in children under 18 years of age.
Who should not take COREG CR?
Do not take COREG CR if you:
- have severe heart failure and require certain intravenous medicines that help support circulation.
- have asthma or other breathing problems.
- have a slow heartbeat or certain conditions that cause your heart to skip a beat (irregular heartbeat).
- have liver problems.
- are allergic to any of the ingredients in COREG CR. See "What are the ingredients in COREG CR?"
What should I tell my doctor before taking COREG CR?
Tell your doctor about all of your medical conditions, including if you:
- have asthma or other lung problems (such as bronchitis or emphysema).
- have problems with blood flow in your feet and legs (peripheral vascular disease). COREG CR can make some of your symptoms worse.
- have diabetes.
- have thyroid problems.
- have a condition called pheochromocytoma.
- have had severe allergic reactions.
- are scheduled for surgery and will be given anesthetic agents.
- are scheduled for cataract surgery and have taken or are currently taking COREG CR.
- are pregnant or trying to become pregnant. It is not known if COREG CR is safe for your unborn baby. You and your doctor should talk about the best way to control your high blood pressure during pregnancy.
- are breastfeeding. It is not known if COREG CR passes into your breast milk. Talk with your doctor about the best way to feed your baby if you are taking COREG CR.
Tell your doctor about all of the medicines you take including prescription and over-the-counter medicines, vitamins, and herbal supplements. COREG CR and certain other medicines can affect each other and cause serious side effects. COREG CR may affect the way other medicines work. Also, other medicines may affect how well COREG CR works.
Know the medicines you take. Keep a list of your medicines and show it to your doctor and pharmacist before you start a new medicine.
How should I take COREG CR?
- Take COREG CR exactly as prescribed. Take COREG CR one time each day with food. It is important that you take COREG CR only one time each day. To lessen possible side effects, your doctor might begin with a low dose and then slowly increase the dose.
- Swallow COREG CR capsules whole. Do not chew or crush COREG CR capsules.
- If you have trouble swallowing COREG CR whole:
- The capsule may be carefully opened and the beads sprinkled over a spoonful of applesauce which should be eaten right away. The applesauce should not be warm.
- Do not sprinkle beads on foods other than applesauce.
- Do not stop taking COREG CR and do not change the amount of COREG CR you take without talking to your doctor.
- If you miss a dose of COREG CR, take your dose as soon as you remember, unless it is time to take your next dose. Take your next dose at the usual time. Do not take 2 doses at the same time.
- If you take too much COREG CR, call your doctor or poison control center right away.
What should I avoıd whıle takıng COREG CR?
COREG CR can cause you to feel dizzy, tired, or faint. Do not drive a car, use machinery, or do anything that needs you to be alert if you have these symptoms.
What are possible side effects of COREG CR?
Serious side effects of COREG CR include:
- chest pain and heart attack if you suddenly stop taking COREG CR. See "What is the most important information I should know about COREG CR?"
- slow heart beat.
- low blood pressure (which may cause dizziness or fainting when you stand up). If these happen, sit or lie down, and tell your doctor right away.
- worsening heart failure. Tell your doctor right away if you have signs and symptoms that your heart failure may be worse, such as weight gain or increased shortness of breath.
- changes in your blood sugar. If you have diabetes, tell your doctor if you have any changes in your blood sugar levels.
- masking (hiding) the symptoms of low blood sugar, especially a fast heartbeat.
-
new or worsening symptoms of peripheral vascular disease.
- leg pain that happens when you walk, but goes away when you rest
- no feeling (numbness) in your legs or feet while you are resting
- cold legs or feet
- masking the symptoms of hyperthyroidism (overactive thyroid), such as a fast heartbeat.
- worsening of severe allergic reactions. Medicines to treat a severe allergic reaction may not work as well while you are taking COREG CR.
- rare but serious allergic reactions (including hives or swelling of the face, lips, tongue, and/or throat that may cause difficulty in breathing or swallowing) have happened in patients who were on COREG or COREG CR. These reactions can be life-threatening. In some cases, these reactions happened in patients who had been on COREG before taking COREG CR.
Common side effects of COREG CR include shortness of breath, weight gain, diarrhea, and tiredness. If you wear contact lenses, you may have fewer tears or dry eyes that can become bothersome.
Call your doctor if you have any side effects that bother you or don't go away.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store COREG CR?
Store COREG CR at less than 86°F (30°C).
Safely throw away COREG CR that is out of date or no longer needed.
Keep COREG CR and all medicines out of the reach of children.
General information about COREG CR
Medicines are sometimes prescribed for conditions other than those described in patient information leaflets. Do not use COREG CR for a condition for which it was not prescribed. Do not give COREG CR to other people, even if they have the same symptoms you have. It may harm them.
This leaflet summarizes the most important information about COREG CR. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about COREG CR that is written for healthcare professionals.
What are the ingredients in COREG CR?
Active ingredient: carvedilol phosphate
Inactive ingredients: crospovidone, hydrogenated castor oil, hydrogenated vegetable oil, magnesium stearate, methacrylic acid copolymers, microcrystalline cellulose, and povidone.
COREG CR capsules come in the following strengths: 10 mg, 20 mg, 40 mg, 80 mg.
What is high blood pressure (hypertension)?
Blood pressure is the force of blood in your blood vessels when your heart beats and when your heart rests. You have high blood pressure when the force is too much. High blood pressure makes the heart work harder to pump blood through the body and causes damage to blood vessels. COREG CR can help your blood vessels relax so your blood pressure is lower. Medicines that lower blood pressure may lower your chance of having a stroke or heart attack.
COREG CR and COREG are trademarks used under license by Waylis Therapeutics LLC.
Manufactured for:
Waylis Therapeutics LLC
Rahway, NJ 07065
Rev. 10/2024
PRINCIPAL DISPLAY PANEL - 10 mg Capsule Bottle Label
10 mg
Waylis
THERAPEUTICS
NDC 80725-383-13
ONCE-A-DAY
DOSING
COREG CR
(carvedilol phosphate)
Extended-release Capsules
30 Capsules
Rx only
PRINCIPAL DISPLAY PANEL - 20 mg Capsule Bottle Label
20 mg
Waylis
THERAPEUTICS
NDC 80725-385-13
ONCE-A-DAY
DOSING
COREG CR
(carvedilol phosphate)
Extended-release Capsules
30 Capsules
Rx only
| COREG CR
carvedilol phosphate capsule, extended release |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| COREG CR
carvedilol phosphate capsule, extended release |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| COREG CR
carvedilol phosphate capsule, extended release |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| COREG CR
carvedilol phosphate capsule, extended release |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Waylis Therapeutics LLC (117678921) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Recipharm Pessac S.A.S. | 266640441 | MANUFACTURE(80725-383, 80725-385, 80725-387, 80725-388) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bora Pharmaceuticals | 205556368 | MANUFACTURE(80725-383, 80725-385, 80725-387, 80725-388) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Future Pak LLC | 087737672 | LABEL(80725-383, 80725-385, 80725-387, 80725-388) | |
More about Coreg CR (carvedilol)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (6)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Generic availability
- Drug class: non-cardioselective beta blockers
- Breastfeeding
- En español