7T Gummy ES Chewable Tablets: Package Insert / Prescribing Info
Package insert / product label
Generic name: acetaminophen
Dosage form: tablet, chewable
Medically reviewed by Drugs.com. Last updated on Sep 16, 2024.
On This Page
7T Gummy ES Chewable Tablets Description
7T Gummy ES is available as a chewable gel or gummy oral dosage form with improved organoleptic properties of taste, softness, chewiness, hardness, and translucency.
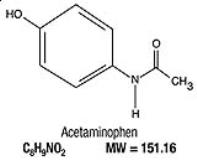
Acetaminophen, 4’-hydroxyacetanilide, a slightly bitter, white, odorless, crystalline powder, is a non-opiate, non-salicylate analgesic and antipyretic. It has the following structural formula:
In addition, each chewable gel contains the following inactive ingredients: hydroxypropyl betadex, seaweed extract (carrageenan), maltitol solution, sugar, sodium citrate, sodium chloride, sucralose, neotame, maltodextrin, glucose syrup, flavor, purified water.
7T Gummy ES Chewable Tablets - Clinical Pharmacology
Mechanism or Action
The precise mechanism of the analgesic properties of acetaminophen is not established but is thought to involve central nervous system actions.
Pharmacodynamics
Therapeutic doses of acetaminophen have negligible effects on the cardiovascular or respiratory systems; however, toxic doses may cause circulatory failure and rapid, shallow breathing.
Pharmacokinetics
Acetaminophen is rapidly absorbed from the gastrointestinal tract and is distributed throughout most body tissues. A small fraction (10 to 25%) of acetaminophen is bound to plasma proteins. The plasma half-life is 1.25 to 3 hours, but may be increased by liver damage and following overdosage. Elimination of acetaminophen is principally by liver metabolism (conjugation) and subsequent renal excretion of metabolites. Acetaminophen is primarily metabolized in the liver by first-order kinetics and involves three principal separate pathways: conjugation with glucuronide; conjugation with sulfate; and oxidation via the cytochrome, P450-dependent, mixed-function oxidase enzyme pathway to form a reactive intermediate metabolite, which conjugates with glutathione and is then further metabolized to form cysteine and mercapturic acid conjugates. The principal cytochrome P450 isoenzyme involved appears to be CYP2E1, with CYP1A2 and CYP3A4 as additional pathways. Approximately 85% of an oral dose appears in the urine within 24 hours of administration, most as the glucuronide conjugate, with small amounts of other conjugates and unchanged drug.
Indications and Usage for 7T Gummy ES Chewable Tablets
7T Gummy ES is used for the temporarily relief of minor aches and pain due to the common cold, flu, headache, sore throat, toothache, and temporarily reduces fever.
Warnings
Keep out of reach of children.
Caution is advised for this formulation with extra strength dosing of acetaminophen due to the easily chewable gels and good taste. This is not a candy and the same caution with every medication should be applied to this product.
Hepatotoxicity
This product contains acetaminophen. Severe liver damage may occur if you take
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a physician or pharmacist.
- 3 or more alcoholic drinks every day while using this product
Acetaminophen has been associated with cases of acute liver failure, at times resulting in liver transplant and death. Most of the cases of liver injury are associated with the use of acetaminophen at doses that exceed 4,000 milligrams per day, and often involve more than one acetaminophen-containing product. The excessive intake of acetaminophen may be intentional to cause self-harm or unintentional as patients attempt to obtain more pain relief or unknowingly take other acetaminophen-containing products.
The risk of acute liver failure is higher in individuals with underlying liver disease and in individuals who ingest alcohol while taking acetaminophen.
Instruct patients to look for acetaminophen or APAP on package labels and not to use more than one product that contains acetaminophen. Instruct patients to seek medical attention immediately upon ingestion of more than 4,000 milligrams of acetaminophen per day, even if they feel well.
Serious Skin Reactions
Rarely, acetaminophen may cause serious skin reactions such as acute generalized exanthematous pustulosis (AGEP), Stevens-Johnson Syndrome (SJS), and toxic epidermal necrolysis (TEN), which can be fatal. Patients should be informed about the signs of serious skin reactions, and use of the drug should be discontinued at the first appearance of skin rash or any other sign of hypersensitivity.
Acetaminophen may cause severe skin reactions. Symptoms may include
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away. Do not use if you are allergic to acetaminophen or any of the inactive ingredients in this product.
Sore throat warning: if sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Precautions
This formulation with extra strength acetaminophen should be taken under the supervision of a physician if:
- taking a blood thinning drug called warfarin
- you or a family member have a history of high blood pressure or you are taking any antihypertensive medications due to the sodium content in each chewable gel
- you or a family member have a history of diabetes or you are taking any diabetes medications due to the sugar content in each chewable gel
Related/similar drugs
Overdosage
Following an acute overdosage, toxicity may result from acetaminophen.
Clinical Presentation:
Dose-dependent, potentially fatal hepatic necrosis is the most serious adverse effect of acetaminophen overdosage. Renal tubular necrosis, hypoglycemic coma and coagulation defects may also occur.
Early symptoms following a potentially hepatotoxic overdose may include: nausea, vomiting, diaphoresis and general malaise. Clinical and laboratory evidence of hepatic toxicity may not be apparent until 48 to 72 hours post-ingestion.
Treatment of Overdose:
Gastric decontamination with activated charcoal should be administered just prior to N-acetylcysteine (NAC) to decrease systemic absorption if acetaminophen ingestion is known or suspected to have occurred within a few hours of presentation. Serum acetaminophen levels should be obtained immediately if the patient presents 4 hours or more after ingestion to assess potential risk of hepatotoxicity; acetaminophen levels drawn less than 4 hours post-ingestion may be misleading. To obtain the best possible outcome, NAC should be administered as soon as possible where impending or evolving liver injury is suspected. Intravenous NAC may be administered when circumstances preclude oral administration.
Vigorous supportive therapy is required in severe intoxication. Procedures to limit the continuing absorption of the drug must be readily performed since the hepatic injury is dose dependent and occurs early in the course of intoxication.
Overdose warning: In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222) Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
7T Gummy ES Chewable Tablets Dosage and Administration
Do not take more than directed (see overdose warning)
Adults and children 12 years and over:
- take 2 chewable gels every 6 hours while symptoms last or as prescribed and directed by a physician
- chew each chewable gel thoroughly before swallowing
- do not take more than 6 chewable gels in 24 hours, unless directed by a physician
- under the supervision of a physician, daily doses up to 8 chewable gels may be used
- do not take for more than 10 days unless prescribed and directed by a physician
Storage and Handling
-
each chewable gel contains: sodium 7.44 mg.
-
each chewable gel contains: sugar 2g.
- store in a cool dry place between 20-25°C (68-77°F).
Child Resistant Container; do not use if printed seal under cap is broken or missing.
Inactive ingredients: hydroxypropyl betadex, seaweed extract (carrageenan), maltitol solution, sugar, sodium citrate, sodium chloride, sucralose, neotame, maltodextrin, glucose syrup, flavor, purified water.
| 7T GUMMY ES
acetaminophen tablet, chewable |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - 7T Pharma LLC (080220022) |
Frequently asked questions
- Can You Take Tramadol with Acetaminophen, Ibuprofen, or Aspirin?
- Acetaminophen vs paracetamol: What do you need to know?
- What's the best medicine for sore throat?
- Can you take Advil & Tylenol together? Safe Dosing Guide
- What is paracetamol / Panadol called in the US?
- Acetaminophen vs Ibuprofen: Which is better?
- What medications cause liver enzymes to be elevated?
- What temperature is considered a fever?
- Is it safe to take acetaminophen every day?
More about 7T Gummy ES Chewable Tablets (acetaminophen)
- Check interactions
- Compare alternatives
- Latest FDA alerts (16)
- Side effects
- Dosage information
- During pregnancy or Breastfeeding
Professional resources
Other brands
Tylenol Arthritis Pain, Ofirmev, Children's Tylenol

