Esketamine (Monograph)
Brand name: Spravato [Web]
Drug class: NMDA Antagonists
Warning
Risk Evaluation and Mitigation Strategy (REMS):
FDA approved a REMS for esketamine to ensure that the benefits outweigh the risks. The REMS may apply to one or more preparations of esketamine and consists of the following: elements to assure safe use and implementation system. See https://www.accessdata.fda.gov/scripts/cder/rems/.
Warning
- Sedation
-
May cause sedation following administration. Administer only in a registered healthcare setting.
-
Monitor patients for ≥2 hours after administration, then assess whether patient is considered clinically stable and ready to leave healthcare setting.
- Dissociation
-
May cause dissociative or perceptual changes following administration. Administer only in a registered healthcare setting.
-
Monitor patients for ≥2 hours after administration, then assess whether patient is considered clinically stable and ready to leave healthcare setting.
- Respiratory Depression
-
May cause respiratory depression. Administer only in a registered healthcare setting.
-
Monitor respiratory status, including pulse oximetry, for ≥2 hours after administration, then assess whether patient is considered clinically stable and ready to leave healthcare setting.
- Abuse and Misuse
-
Potential for abuse and misuse.
-
Consider risks and benefits of prescribing esketamine prior to use in patients at higher risk of abuse. Monitor patients for signs and symptoms of abuse and misuse.
- Suicidality
-
Antidepressants increased risk of suicidal thinking and behavior (suicidality) compared with placebo in pediatric and young adult patients in short-term studies. Esketamine is not labeled for use in pediatric patients.
-
Closely monitor all patients receiving antidepressants for clinical worsening and for emergence of suicidality.
Introduction
Antidepressant; N-methyl-d-aspartate (NMDA) receptor antagonist and S-enantiomer of racemic ketamine.
Uses for Esketamine
Esketamine is used intranasally for depression. Esketamine has not demonstrated effectiveness in preventing suicide or in reducing suicidal thoughts and behaviors (suicidality). Esketamine use does not preclude the need for hospitalization if clinically warranted, even if patients experience improvement after an initial dose of esketamine. Esketamine nasal solution is not labeled for use as an anesthetic agent and the manufacturer states that safety and efficacy of the drug for this use have not been established.
Treatment-resistant Depression
Used for treatment-resistant depression in adults, as monotherapy or in combination with an oral antidepressant.
Although the definition has varied, treatment-resistant depression is often defined as the failure of ≥2 trials of first-line antidepressants given in an adequate dosage for an adequate duration of therapy.
Intranasal esketamine substantially improved depressive symptoms within 24 hours of administration in a short-term clinical trial and prolonged time to relapse in a longer-term maintenance trial.
Guidelines from the American Psychiatric Association (APA) and other experts recommend that patients with treatment-resistant depression switch to another antidepressant (e.g., from one SSRI to another or to a tricyclic antidepressant or MAO inhibitor), augment with a nonantidepressant medication (e.g., lithium, antipsychotic), add or switch to psychotherapy, or use other nonpharmacologic treatments (e.g., transcranial magnetic stimulation, electroconvulsive therapy). The Department of Veterans Affairs and Department of Defense guidelines for the management of major depressive disorder state that esketamine can be considered for augmentation in patients not responsive to several adequate pharmacologic trials.
Depressive Symptoms in Major Depressive Disorder with Acute Suicidality
Used in combination with an oral antidepressant for treatment of depressive symptoms in adults with major depressive disorder with acute suicidal ideation or behavior.
Intranasal esketamine substantially improved depressive symptoms within 24 hours of administration in 2 short-term clinical trials; the treatment difference was noted starting 4 hours after administration.
Esketamine Dosage and Administration
General
Pretreatment Screening
-
Monitor BP prior to administr. If baseline BP is elevated (i.e., systolic BP >140 or diastolic BP >90 mm Hg), consider the risks of short-term increases in BP and the benefits of treatment. Do not administer in patients in whom an increase in BP or intracranial pressure would constitute a serious hazard.
-
Because of the risk of dissociative effects, assess patients with psychosis carefully prior to treatment; initiate therapy in such patients only if the benefit outweighs the risk.
-
Assess risk for abuse or misuse.
Patient Monitoring
-
Monitor BP approximately 40 minutes after administration and as clinically indicated thereafter until BP values decline. If BP is decreasing and the patient appears clinically stable for ≥2 hours, may discharge patient at the end of the post-dose monitoring period; if not, continue patient monitoring. If BP remains elevated, promptly consult clinicians experienced in BP management; patients experiencing symptoms of hypertensive crisis or hypertensive encephalopathy should be referred for emergency care.
-
Because of the risks of sedation and dissociation, monitor patients for at least 2 hours after each treatment session, then assess to determine if the patient is clinically stable and ready to leave the healthcare setting. Closely monitor patients receiving esketamine and CNS depressants concomitantly.
-
Monitor for changes in respiratory status, including pulse oximetry, for at least 2 hours at each treatment session, then assess to determine when the patient is considered clinically stable and ready to leave the healthcare setting.
-
Monitor patients for possible worsening of depression or emergence of suicidality, especially at the beginning of therapy or during periods of dosage adjustment.
-
Monitor for urinary tract and bladder symptoms (e.g., dysuria, urinary frequency or urgency, nocturia) during treatment. If urinary symptoms occur, patients should be evaluated by an appropriate healthcare provider as clinically warranted.
-
Monitor for signs and symptoms of physical dependence following discontinuance of therapy.
Dispensing and Administration Precautions
-
The Institute for Safe Medication Practices (ISMP) includes Spravato (esketamine hydrochloride) and Steglatro (ertugliflozin) on the ISMP List of Confused Drug Names, and recommends special safeguards to ensure the accuracy of prescriptions for these drugs.
-
Handle esketamine nasal spray devices with adequate security and accountability, and ensure disposal is in accordance with the facility's procedures and federal, state, and local regulations for C-III drug products.
REMS
-
Only available through the Spravato REMS program.
-
Healthcare settings and pharmacies must be certified with the program before they can supervise patient self-administration of esketamine nasal spray and dispense the drug; patients must be enrolled in the program to receive treatment. Esketamine nasal spray must be administered under the direct observation of a healthcare provider in a certified healthcare setting.
-
Further information about the Spravato REMS program, including a list of certified pharmacies, is available at [Web] or at 855-382-6022.
Other General Considerations
-
If use of an intranasal corticosteroid or decongestant is required on the same day as treatment with esketamine nasal spray, these products should be administered ≥1 hour prior to esketamine.
-
Nausea and vomiting may occur following intranasal administration; advise patients to avoid eating food for ≥2 hours and to avoid drinking liquids for ≥30 minutes prior to administration.
-
Prior to administration, instruct patients not to engage in potentially hazardous activities (e.g., driving a motor vehicle, operating machinery) until the next day after a restful sleep. Patients should also arrange for transportation home following each esketamine treatment session.
Administration
Intranasal Administration
Intended for patient self-administration under the direct supervision of a healthcare provider. A treatment session consists of intranasal administration of esketamine and post-administration observation under supervision.
Instruct patients on use of the nasal spray device and advise them to read the patient instructions for use provided by the manufacturer.
Device Preparation
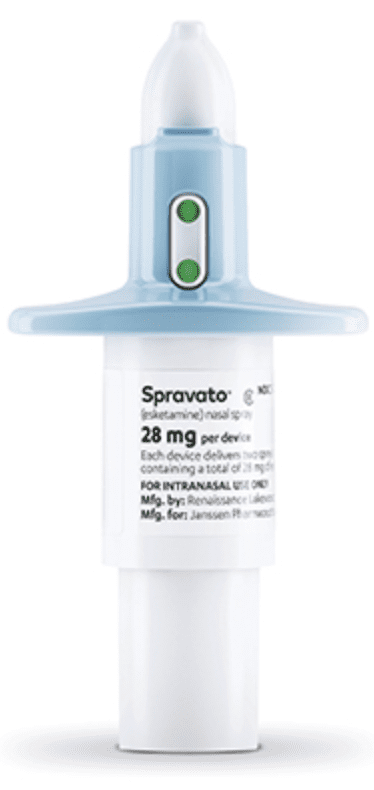
Esketamine nasal solution is commercially available in kits containing either 2 (56-mg dose kit) or 3 (84-mg dose kit) stoppered glass vials within a nasal spray device. Each device delivers 14 mg of esketamine per spray and delivers 2 sprays (1 spray per nostril) per device. Therefore, a 56-mg dose requires 2 devices, and an 84-mg dose requires 3 devices.
An indicator on the device displays one green light for each spray remaining in the device. Prior to administration, a healthcare provider should confirm the number of nasal spray devices required for the dose and check that each device indicator displays 2 green dots.
To prevent loss of the drug, do not prime esketamine nasal spray devices before use.
Administration Procedure
Before initial spray from the first device at each treatment session, blow the nose to clear nasal passages. During administration, patients should recline their head to about 45 degrees to help keep the solution inside the nose.
Insert the tip of the device straight into one nostril; the nose rest should touch the skin between the nostrils. While holding the other nostril closed, concurrently inspire through the nose while pushing on the plunger to activate the spray. Following actuation of the spray, sniff gently to keep the solution in the nose. Repeat this procedure for the other nostril. If liquid drips out of the nostril, dab the nose with a tissue.
Repeat procedure for each device until the full dose has been administered. Following administration of each device (i.e., 2 sprays), patients should rest in a comfortable position (preferably semi-reclined) for 5 minutes.
Following use, the healthcare provider should check each device to ensure that both sprays have been delivered; if a green dot appears in the device indicator, the patient should spray again into the second nostril.
Properly dispose of used devices in accordance with the facility's procedures and federal, state, and local regulations for controlled substance disposal.
Dosage
Available as esketamine hydrochloride; dosage expressed in terms of esketamine.
Adults
Treatment-resistant Depression
Intranasal
Administer as monotherapy or in combination with an oral antidepressant.
Induction phase: Initially, 56 or 84 mg (adjust dosage based on efficacy and tolerability) administered twice weekly during weeks 1–4.
Maintenance phase: 56 or 84 mg once weekly during weeks 5–8, then 56 or 84 mg administered every 2 weeks or once weekly during week 9 and afterward. Individualize dosing frequency to the least frequent dosing interval that maintains remission or response.
Assess clinical benefit at the end of the induction phase (i.e., week 4) to determine need for continued therapy.
In short- and long-term efficacy trials, approximately one-third of patients received 56-mg doses and two-thirds received 84-mg doses of the drug.
If a treatment session is missed and there is no worsening of depressive symptoms, continue the current dosing schedule. If a treatment session is missed during the maintenance phase and worsening of depression occurs, may increase dosing frequency back to patient's previous dosing schedule (i.e., every 2 weeks to once weekly, once weekly to twice weekly) based on clinical judgment.
Depressive Symptoms in Major Depressive Disorder with Acute Suicidal Ideation or Behavior
Intranasal
Administer in combination with an oral antidepressant.
Initially, 84 mg twice weekly for 4 weeks; may reduce to 56 mg twice weekly based on tolerability.
After 4 weeks, evidence of therapeutic benefit should be evaluated to determine need for continued treatment.
Use beyond 4 weeks has not been systemically evaluated in this patient population.
Special Populations
Hepatic Impairment
Mild hepatic impairment: No specific dosage recommendations.
Moderate hepatic impairment: No specific dosage adjustment recommended; may require prolonged monitoring for adverse effects.
Severe hepatic impairment: Use not recommended.
Renal Impairment
No specific dosage recommendations.
Geriatric Patients
No specific dosage recommendations.
Cautions for Esketamine
Contraindications
-
Aneurysmal vascular disease (including that affecting the thoracic and abdominal aorta and intracranial and peripheral arterial vessels) or arteriovenous malformation.
-
History of intracerebral hemorrhage.
-
Hypersensitivity to esketamine, ketamine, or any excipient in the formulation.
Warnings/Precautions
Warnings
Sedation
May cause sedation or loss of consciousness (see Boxed Warning).
Monitor patients for ≥2 hours at each treatment session for sedation, then assess patients to determine if they are clinically stable and ready to leave healthcare setting.
Closely monitor patients receiving CNS depressants concurrently.
Dissociation
May cause dissociative effects (e.g., derealization, depersonalization) and perceptual changes (e.g., distortion of time and space, illusions); these adverse effects appear to be dose related (see Boxed Warning). In clinical trials, dissociation was transient and occurred on the day of treatment.
Carefully assess patients with psychosis prior to treatment; initiate therapy only if the benefits outweigh the risks.
Monitor patients for dissociative effects for ≥2 hours at each treatment session, then assess patients to determine if they are clinically stable and ready to leave healthcare setting.
Respiratory Depression
Respiratory depression, including respiratory arrest, reported in postmarketing experience (see Boxed Warning).
Monitor respiratory status (including pulse oximetry) for ≥2 hours at each treatment session, then assess patients to determine if they are clinically stable and ready to leave healthcare setting.
Abuse and Misuse
Potential for abuse or misuse (see Boxed Warning); abuse potential appears to be similar to that of IV ketamine (also a schedule III [C-III] drug). Esketamine produces a variety of symptoms, including anxiety, dysphoria, disorientation, insomnia, flashbacks, hallucinations, and feelings of floating, detachment, and being “spaced out”.
Physical dependence reported with prolonged ketamine use and withdrawal symptoms reported following discontinuance of frequently used large doses of ketamine given over prolonged periods. Although withdrawal symptoms not observed after cessation of esketamine therapy, such symptoms are likely to occur if similarly abused. Tolerance reported with prolonged use of ketamine; similar tolerance expected with prolonged use of esketamine.
Assess patient's risk for abuse or misuse prior to prescribing esketamine, and monitor patients for development of these behaviors or conditions, including drug-seeking behavior, during therapy.
Individuals with a history of drug abuse or dependence may be at increased risk for abuse and misuse. Careful consideration is advised prior to esketamine use in individuals with a history of substance use disorder, including alcohol.
Monitor patients for signs and symptoms of physical dependence following discontinuance of therapy.
Handle esketamine nasal spray devices with adequate security and accountability, and ensure disposal is in accordance with facility's procedures and federal, state, and local regulations for C-III drug products.
Suicidality
Possible worsening of depression and/or the emergence of suicidal ideation and behavior (suicidality; see Boxed Warning). Increased risk of suicidality in pediatric patients and young adults (18–24 years of age) in short-term, placebo-controlled studies receiving antidepressants for major depressive disorder and other indications. Risk of suicidality was not increased in adults >24 years of age and apparently was reduced in adults ≥65 years of age with antidepressant therapy compared with placebo. It is not known whether risk of suicidality in children, adolescents, and young adults extends to longer-term use (i.e., >4 months) of antidepressants; however, substantial evidence indicates that antidepressants delay the recurrence of depression and that depression itself is a risk factor for suicidality.
Monitor all patients being treated with antidepressants for any indication for clinical worsening and emergence of suicidality, particularly during initiation of therapy (i.e., the first few months) and during periods of dosage adjustments. Advise family members and caregivers of patients being treated with antidepressants to monitor patients for changes in behavior and to alert clinician if such changes occur.
Consider changing therapeutic regimen, including possible discontinuance of esketamine and/or the concomitant oral antidepressant, in patients whose depression is persistently worse or who are experiencing emergent suicidality.
Esketamine is not labeled for use in pediatric patients.
Other Warnings and Precautions
Increases in BP
Increases in SBP and/or DBP can occur at all recommended doses. Substantial increases in BP could occur after any dose even if smaller BP effects were observed with previous doses. BP increases peak approximately 40 minutes after intranasal administration and last for approximately 4 hours.
Assess BP prior to each treatment session, approximately 40 minutes after administration of the dose, and as clinically warranted for ≥2 hours following administration until values decline. If baseline BP is elevated (i.e., SBP ≥140 mm Hg or DBP ≥90 mm Hg) prior to administration, consider potential benefits and risks of esketamine when deciding whether to delay therapy or administer the drug.
If BP is decreasing following esketamine administration and patient appears clinically stable for ≥2 hours, may discharge from healthcare setting; otherwise, continue monitoring. If BP remains elevated, promptly consult clinicians experienced in BP management. If patient experiences symptoms of hypertensive crisis (e.g., chest pain, shortness of breath) or hypertensive encephalopathy (e.g., sudden severe headache, visual disturbances, seizures, diminished consciousness, focal neurologic deficits), refer for emergency care.
Patients with a history of hypertensive encephalopathy are at increased risk for developing encephalopathy with even small BP increases; monitor such patients more intensively, including more frequent BP monitoring and symptom assessment.
Contraindicated in patients in whom an increase in BP or intracranial pressure would constitute a serious hazard (e.g., aneurysmal vascular disease, arteriovenous malformation, history of intracerebral hemorrhage). Carefully assess patients with other cardiovascular or cerebrovascular conditions to determine whether potential benefits of esketamine treatment outweigh the risks.
Closely monitor BP if esketamine is used concomitantly with psychostimulants or monoamine oxidase (MAO) inhibitors.
Cognitive Impairment
May cause short-term impairment in attention, judgment, thinking, reaction speed, and motor skills.
In a clinical study, a greater decline in cognitive function was observed and greater mental effort was required to complete cognitive tests compared with placebo at 40 minutes post-dose; however, cognitive performance and mental effort were comparable to placebo groups at 2 hours post-dose. Esketamine also was associated with increased sleepiness at 40 minutes and 2 hours post-dose, but was comparable to placebo by 4 hours post-dose.
In 1- and 3-year, long-term, open-label clinical trials, cognitive functioning remained stable over time.
Impaired Ability to Drive or Operate Machinery
May impair the ability to drive a motor vehicle or operate machinery. Following treatment, patients should not engage in potentially hazardous activities requiring full mental alertness and motor coordination, such as driving or operating machinery, until the next day after a restful sleep. Patients should also arrange transportation home following each esketamine treatment session.
Ulcerative or Interstitial Cystitis
Ulcerative or interstitial cystitis reported with long-term, off-label use or misuse/abuse of ketamine. In clinical studies, lower urinary tract symptoms (e.g., pollakiuria, dysuria, urgency, nocturia, cystitis) reported more frequently in patients receiving intranasal esketamine compared with placebo. However, interstitial cystitis not reported in any studies, including those of up to 1 year duration.
Monitor patients for urinary tract and bladder symptoms (e.g., dysuria, urinary frequency or urgency, nocturia) during therapy. If such symptoms occur, refer patient to an appropriate healthcare provider for evaluation as clinically warranted.
Embryo-fetal Toxicity
Based on ketamine data in animals, esketamine may cause fetal harm if administered to pregnant women. Clinical relevance in humans treated with intranasal esketamine at the recommended dosage not known.
Repeated or prolonged use of drugs that block NMDA receptors, including ketamine and esketamine, during the third trimester of pregnancy may result in adverse neurodevelopmental effects in the fetus.
Not recommended for use during pregnancy. Women of reproductive potential should consider pregnancy planning and contraception during therapy. If patient becomes pregnant while receiving the drug, discontinue therapy and apprise of potential fetal hazard.
Specific Populations
Pregnancy
Insufficient experience in pregnant women; may cause fetal harm. Not recommended for use during pregnancy. If patient becomes pregnant during treatment, discontinue esketamine and counsel patient on potential risk to fetus.
National Pregnancy Registry for Antidepressants: 844-405-6185 or [Web].
Lactation
Distributed into human milk. Effects on the nursing infant or milk production not known. Potential for neurotoxicity in nursing infants; breast-feeding during therapy not recommended.
Females and Males of Reproductive Potential
May cause embryo-fetal harm when administered to a pregnant woman. Consider pregnancy planning and prevention for females of reproductive potential during treatment.
Pediatric Use
Safety and efficacy not established.
Increased risk of suicidality in children and adolescents receiving antidepressants for major depressive disorder and other indications.
Repeated or prolonged use of drugs that block NMDA receptors, including ketamine and esketamine, in children ≤3 years of age may adversely affect neurodevelopment. In animals, use of drugs that block NMDA receptors leads to neuronal and oligodendrocyte cell loss and alterations in synaptic morphology and neurogenesis in the developing brain. Clinical relevance of these findings to humans treated with intranasal esketamine at the recommended dosage not known.
Geriatric Use
No overall differences in safety profile observed between geriatric patients ≥65 years of age and younger adults in clinical studies.
In a clinical trial in patients ≥65 years of age with treatment-resistant depression, intranasal esketamine in conjunction with a newly initiated oral antidepressant reduced symptoms of depression compared with intranasal placebo plus an oral antidepressant; however, difference was not statistically significant.
Hepatic Impairment
Mild hepatic impairment (Child-Pugh class A): Slightly increased peak plasma concentrations and exposure; these changes not considered clinically important.
Moderate hepatic impairment (Child-Pugh class B): Increased systemic exposure and prolonged half-life. Monitoring for possible adverse effects may be needed for a longer period of time.
Severe hepatic impairment (Child-Pugh class C): Not studied; use not recommended.
Renal Impairment
Slightly increased systemic exposure in individuals with renal impairment; not considered clinically important.
Not studied in individuals requiring dialysis to date.
Common Adverse Effects
Treatment-resistant depression (≥5% and at least twice that of placebo): dissociation, dizziness, nausea, sedation, vertigo, hypoesthesia, anxiety, lethargy, increased blood pressure, vomiting, drunk feeling, headache.
Depressive symptoms in major depressive disorder with acute suicidal ideation or behavior (≥5% and at least twice that of placebo): dissociation, dizziness, sedation, increased blood pressure, hypoesthesia, vomiting, euphoric mood, vertigo.
Drug Interactions
Principally metabolized by CYP2B6 and CYP3A4 and, to a lesser extent, by CYP2C9 and CYP2C19. Major active metabolite noresketamine also metabolized via CYP-mediated pathways.
Esketamine modestly induces CYP2B6 and CYP3A4 and noresketamine weakly and reversibly inhibits CYP3A4 in vitro.
Drugs Affecting or Metabolized by Hepatic Microsomal Enzymes
Inhibitors or inducers of CYP2B6 or CYP3A4: Clinically important pharmacokinetic interactions unlikely; dosage adjustment of esketamine not necessary.
Substrates of CYP2B6 or CYP3A: Clinically important pharmacokinetic interactions unlikely.
Specific Drugs
|
Drug |
Interaction |
Comments |
|---|---|---|
|
Bupropion |
No clinically important effect on the pharmacokinetics of bupropion (CYP2B6 substrate) or hydroxybupropion |
|
|
Clarithromycin |
Clarithromycin (strong CYP3A4 inhibitor) slightly increased peak plasma concentrations and AUC of esketamine; not considered clinically important |
Esketamine dosage adjustment not necessary |
|
CNS depressants (e.g., alcohol, benzodiazepines, opiates) |
Possible additive CNS depression (e.g., sedation) |
Monitor closely for sedation during concurrent use |
|
Corticosteroids, intranasal (e.g., mometasone furoate) |
Esketamine pharmacokinetics not substantially affected when intranasal mometasone furoate administered 1 hour prior to intranasal esketamine |
Esketamine dosage adjustment not necessary; if intranasal corticosteroid required on an esketamine dosing day, administer the corticosteroid ≥1 hour prior to intranasal esketamine |
|
Decongestants, intranasal (e.g., oxymetazoline) |
Esketamine pharmacokinetics not substantially affected when intranasal oxymetazoline administered 1 hour prior to intranasal esketamine |
Esketamine dosage adjustment not necessary; if intranasal decongestant required on an esketamine dosing day, administer the decongestant ≥1 hour prior to intranasal esketamine |
|
MAO inhibitors |
Possible increased BP |
Closely monitor BP |
|
Midazolam |
No clinically important effect on the pharmacokinetics of midazolam (a CYP3A substrate) |
|
|
Oral contraceptives |
Esketamine not expected to affect systemic exposure to ethinyl estradiol |
|
|
Psychostimulants (e.g., amphetamines, armodafinil, methylphenidate, modafinil) |
Possible increased BP |
Closely monitor BP |
|
Rifampin |
Rifampin (strong CYP inducer) decreased esketamine peak plasma concentrations and AUC; not considered clinically important |
Dosage adjustment not necessary |
|
Ticlopidine |
Ticlopidine (CYP2B6 inhibitor) increased esketamine peak plasma concentrations and AUC; not considered clinically important |
Dosage adjustment not necessary |
Esketamine Pharmacokinetics
Absorption
Bioavailability
Rapidly absorbed following intranasal administration; peak concentrations attained in 20–40 minutes following last nasal spray of a treatment session.
Absolute bioavailability: Approximately 48% following intranasal administration.
Peak concentrations and AUC increase in a less than dose-proportional manner between 28–84 mg and in a nearly dose-proportional manner between 56–84 mg.
Interpatient variation in peak plasma concentrations and exposure: 27–66 and 18–45%, respectively.
Intrapatient variation in peak plasma concentrations and exposure: Approximately 15 and 10%, respectively.
Onset
Increases in BP peak approximately 40 minutes following intranasal administration.
Duration
Increases in BP last approximately 4 hours following intranasal administration.
Special Populations
Geriatric patients: Increased peak plasma concentrations and AUC.
Mild hepatic impairment (Child-Pugh class A): Slightly increased peak plasma concentrations and exposure; not considered clinically important.
Moderate hepatic impairment (Child-Pugh class B): Peak plasma concentrations and exposure increased by approximately 8% and 103%, respectively, compared with individuals with normal hepatic function.
Mild to severe renal impairment (Clcr 5–77 mL/minute; not requiring dialysis): Slightly increased systemic exposure; not considered clinically important.
Distribution
Extent
Crosses the blood-brain barrier; brain-to-plasma ratio of noresketamine (the principal active metabolite of esketamine) is 4–6 times lower than that of esketamine.
Distributes into human milk.
Plasma Protein Binding
Approximately 43–45%.
Elimination
Metabolism
Metabolized principally by CYP2B6 and CYP3A4 and, to a lesser extent, by CYP2C9 and CYP2C19 to its principal active metabolite noresketamine.
Noresketamine also metabolized via CYP-mediated pathways and certain metabolites undergo glucuronidation.
Elimination Route
Excreted in urine (≥78%) and feces (≤2%) as metabolites.
<1% excreted unchanged in urine.
Half-life
Following intranasal administration, plasma esketamine and noresketamine concentrations decline in a biphasic manner, with a rapid initial phase (2–4 or 4 hours for esketamine or noresketamine, respectively) and a longer elimination phase (mean terminal half-life of 7–12 or 8 hours for esketamine or noresketamine, respectively).
Special Populations
Moderate hepatic impairment (Child-Pugh class B): Increased half-life of esketamine (18.7 hours).
Pharmacokinetics not impacted by sex or total body weight (>39 to 170 kg).
Stability
Storage
Intranasal
Solution
20–25°C (may be exposed to 15–30°C).
Actions
-
Esketamine is the S-enantiomer of racemic ketamine, a derivative of phencyclidine (PCP). The drug is a nonselective, noncompetitive antagonist of the NMDA receptor (an inotropic glutamate receptor). Esketamine has a higher affinity for the NMDA receptor than the R-enantiomer of racemic ketamine.
-
Precise mechanism of antidepressant activity not fully established. Thought to preferentially block NMDA receptors on inhibitory γ-aminobutyric acid (GABA)-ergic interneurons and transiently enhance the activity of glutamatergic neurons, leading to improved synaptic connectivity.
-
Esketamine's mechanism of antidepressant activity is thought to be similar to that of ketamine and is not thought to directly involve serotonin, norepinephrine, or dopamine reuptake, nor is it believed to directly involve µ opioid receptor stimulation.
Advice to Patients
-
Advise patients to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
-
Esketamine nasal spray is only available through the Spravato REMS program. Advise patients that they must be enrolled in the REMS program prior to drug administration. Inform patients that esketamine treatment must be administered under the direct supervision of a healthcare provider and that patients must be monitored by a healthcare provider for ≥2 hours following intranasal administration of the drug.
-
Advise patients of the risk of sedation, dissociative symptoms, disturbances in perception, dizziness, vertigo, anxiety, and/or respiratory depression. Advise patients to notify a healthcare provider immediately if they feel like they cannot stay awake or feel like they may lose consciousness. Inform patients that they will be monitored by a healthcare provider until these potential adverse effects resolve.
-
Advise patients that esketamine is a federally controlled substance because of its potential for abuse, misuse, and dependence.
-
Advise patients of the risk of suicidal thoughts and behaviors (suicidality). Advise patients and caregivers of the importance of being alert to emergence of suicidality, especially early during therapy or during periods of dosage adjustments.
-
Inform patients of the risk of BP increases. Inform patients that they may require observation by a healthcare provider until the BP elevation resolves. Advise patients to notify a healthcare provider immediately if they experience chest pain, shortness of breath, sudden severe headache, changes in vision, or seizures after administration.
-
Advise patients that nausea and vomiting may occur during esketamine therapy. Advise patients not to eat for ≥2 hours before administering esketamine nasal spray and to avoid drinking liquids for ≥30 minutes before administering the drug.
-
Inform patients that esketamine may impair their ability to drive or operate machinery. Following a treatment session, advise patients not to drive, operate machinery, or engage in potentially hazardous activities requiring full mental alertness and motor coordination until the next day after a restful sleep. Advise patients that they will need someone to drive them home after each treatment session.
-
Advise female patients of reproductive potential to inform their clinician if they are or plan to become pregnant or plan to breast-feed. Inform female patients of reproductive potential of the potential fetal risk and inform patients who become pregnant while taking esketamine about the existence of the national pregnancy registry for antidepressants. Advise patients to avoid breast-feeding during esketamine therapy.
-
Advise patients to inform clinicians of existing or contemplated concomitant therapy, including prescription, OTC, and recreational drugs and dietary or herbal supplements, as well as any concomitant or past illnesses (e.g., cardiovascular or cerebrovascular disease, hepatic disease, psychosis, substance abuse or dependence).
-
Advise patients of other important precautionary information.
Additional Information
The American Society of Health-System Pharmacists, Inc. represents that the information provided in the accompanying monograph was formulated with a reasonable standard of care, and in conformity with professional standards in the field. Readers are advised that decisions regarding use of drugs are complex medical decisions requiring the independent, informed decision of an appropriate health care professional, and that the information contained in the monograph is provided for informational purposes only. The manufacturer’s labeling should be consulted for more detailed information. The American Society of Health-System Pharmacists, Inc. does not endorse or recommend the use of any drug. The information contained in the monograph is not a substitute for medical care.
Preparations
Excipients in commercially available drug preparations may have clinically important effects in some individuals; consult specific product labeling for details.
Please refer to the ASHP Drug Shortages Resource Center for information on shortages of one or more of these preparations.
Subject to control under the Federal Controlled Substances Act of 1970 as a schedule III (C-III) drug.
Distribution of esketamine is restricted. Further information about the Spravato REMS program, including a list of certified pharmacies, is available at [Web] or at 855-382-6022.
|
Routes |
Dosage Forms |
Strengths |
Brand Names |
Manufacturer |
|---|---|---|---|---|
|
Nasal |
Solution |
14 mg (of esketamine) per metered spray (14 mg/0.1 mL) |
Spravato 56 mg Dose Kit (C-III; contains two 28-mg nasal spray devices) |
Janssen |
|
Spravato 84 mg Dose Kit (C-III; contains three 28-mg nasal spray devices) |
Janssen |
AHFS DI Essentials™. © Copyright 2025, Selected Revisions July 10, 2025. American Society of Health-System Pharmacists, Inc., 4500 East-West Highway, Suite 900, Bethesda, Maryland 20814.
Reload page with references included
Related/similar drugs
Frequently asked questions
More about esketamine
- Check interactions
- Compare alternatives
- Reviews (138)
- Side effects
- Dosage information
- During pregnancy
- Drug class: miscellaneous antidepressants
- Breastfeeding
- En español