Tri-Nymyo Dosage
Generic name: NORGESTIMATE 0.18mg, ETHINYL ESTRADIOL 0.035mg; NORGESTIMATE 0.215mg, ETHINYL ESTRADIOL 0.035mg; NORGESTIMATE 0.25mg, ETHINYL ESTRADIOL 0.035mg;
Dosage form: tablets
Drug class: Contraceptives
Medically reviewed by Drugs.com. Last updated on Nov 28, 2024.
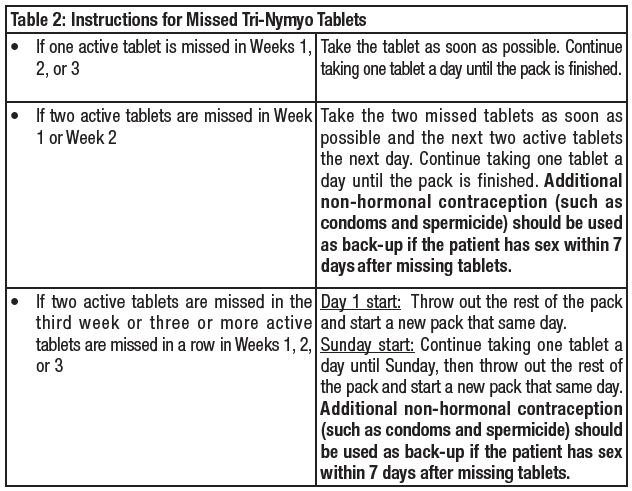
How to Start Tri-Nymyo
Tri-Nymyo is dispensed in 28-tablet blister. Tri-Nymyo may be started using either a Day 1 start or a Sunday start (see Table 1). The plastic compact is pre-set for a Sunday start. Day 1 Start day-label stickers are available. For the first cycle of a Sunday Start regimen, an additional method of contraception should be used until after the first 7 consecutive days of administration.
How to Take Tri-Nymyo
Starting Tri-Nymyo after Abortion or Miscarriage
First-trimester
- After a first-trimester abortion or miscarriage, Tri-Nymyo may be started immediately. An additional method of contraception is not needed if Tri-Nymyo is started immediately.
- If Tri-Nymyo is not started within 5 days after termination of the pregnancy, the patient should use additional non-hormonal contraception (such as condoms and spermicide) for the first seven days of her first cycle pack of Tri-Nymyo.
- Do not start until 4 weeks after a second-trimester abortion or miscarriage, due to the increased risk of thromboembolic disease. Start Tri-Nymyo, following the instructions in Table 1 for Day 1 or Sunday start, as desired. If using Sunday start, use additional non-hormonal contraception (such as condoms and spermicide) for the first seven days of the patient's first cycle pack of Tri-Nymyo.
Starting Tri-Nymyo after Childbirth
- Do not start until 4 weeks after delivery, due to the increased risk of thromboembolic disease. Start contraceptive therapy with Tri-Nymyo following the instructions in Table 1 for women not currently using hormonal contraception.
- Tri-Nymyo is not recommended for use in lactating women.
- If the woman has not yet had a period postpartum, consider the possibility of ovulation and conception occurring prior to use of Tri-Nymyo..
If the patient starts pill-taking on Sunday,the first active pill should be taken on the first Sunday after the patient's menstrual period begins.Remove the first active pill at the top of the dispenser (Sunday) by pressing the pill through the blister foil.
If the patient will start pill-taking on "Day 1," place a day-label sticker on the compact which starts with the day of the week the patient will take the first pill. Remove the first active pill at the top of the dispenser (Day 1) by pressing the pill through the blister foil.
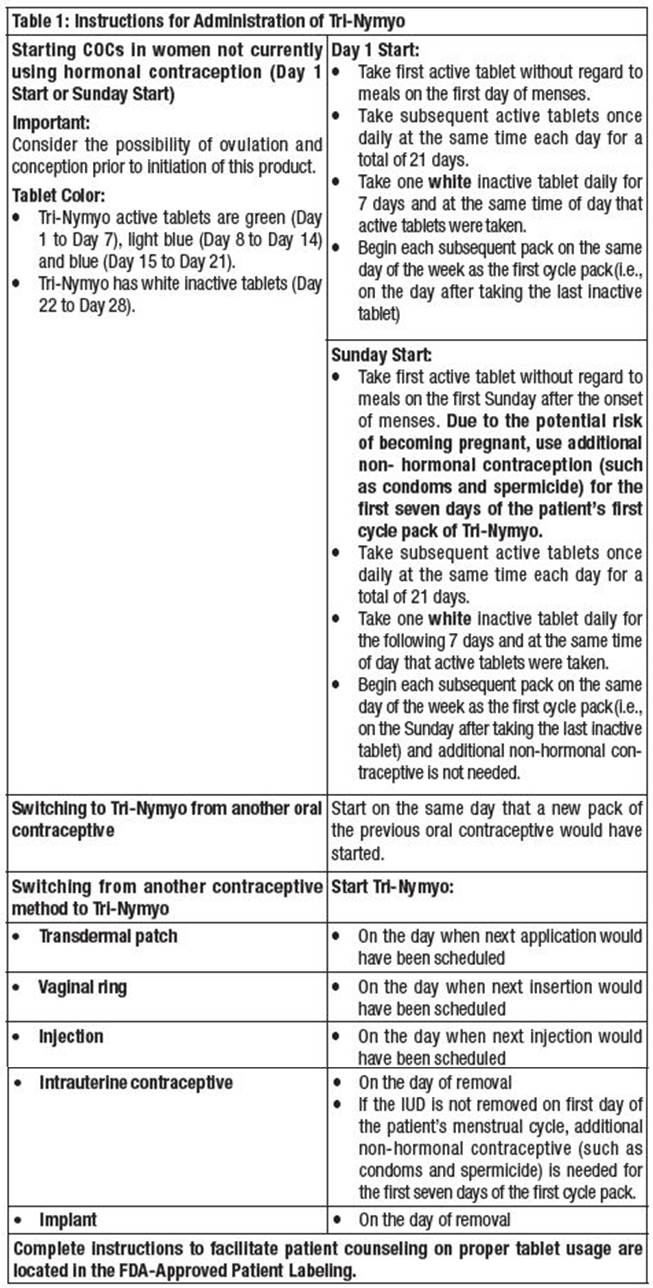
Advice in Case of Gastrointestinal Disturbances
In case of severe vomiting or diarrhea, absorption may not be complete and additional contraceptive measures should be taken. If vomiting or diarrhea occurs within 3 to 4 hours after taking an active tablet, handle this as a missed tablet.
Tri-Nymyo Use for Acne
The timing of initiation of dosing with Tri-Nymyo for acne should follow the guidelines for use of Tri-Nymyo as an oral contraceptive. Consult the DOSAGE AND ADMINISTRATION section (2.1) for instructions.
More about Tri-Nymyo (ethinyl estradiol / norgestimate)
- Check interactions
- Compare alternatives
- Drug images
- Side effects
- During pregnancy
- Drug class: contraceptives
Patient resources
Other brands
Sprintec, Ortho Tri-Cyclen, Tri-Sprintec, Ortho Tri-Cyclen Lo, ... +6 more
Professional resources
Other brands
Sprintec, Estarylla, Ortho Tri-Cyclen, Tri-Sprintec, ... +21 more
Related treatment guides
See also:
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.