Steqeyma Dosage
Generic name: ustekinumab 45mg in 0.5mL
Dosage form: injection, solution
Drug class: Interleukin inhibitors
Medically reviewed by Drugs.com. Last updated on Apr 21, 2025.
Recommended Dosage in Plaque Psoriasis
Subcutaneous Adult Dosage Regimen
- For patients weighing 100 kg or less, the recommended dosage is 45 mg initially and 4 weeks later, followed by 45 mg every 12 weeks.
- For patients weighing more than 100 kg, the recommended dosage is 90 mg initially and 4 weeks later, followed by 90 mg every 12 weeks.
In subjects weighing more than 100 kg, 45 mg was also shown to be efficacious. However, 90 mg resulted in greater efficacy in these subjects.
Subcutaneous Pediatric Dosage Regimen
Administer STEQEYMA subcutaneously at Weeks 0 and 4, then every 12 weeks thereafter.
The recommended dose of STEQEYMA for pediatric patients (6-17 years old) with plaque psoriasis based on body weight is shown below (Table 1).
| Body Weight of Patient at the Time of Dosing | Recommended Dose |
|---|---|
| 60 kg to 100 kg | 45 mg |
| more than 100 kg | 90 mg |
There is no dosage form for STEQEYMA that allows weight-based dosing for pediatric patients below 60 kg.
Recommended Dosage in Psoriatic Arthritis
Subcutaneous Adult Dosage Regimen
- The recommended dosage is 45 mg initially and 4 weeks later, followed by 45 mg every 12 weeks.
- For patients with co-existent moderate-to-severe plaque psoriasis weighing more than 100 kg, the recommended dosage is 90 mg initially and 4 weeks later, followed by 90 mg every 12 weeks.
Subcutaneous Pediatric Dosage Regimen
Administer STEQEYMA subcutaneously at Weeks 0 and 4, then every 12 weeks thereafter.
The recommended dose of STEQEYMA for pediatric patients (6 to 17 years old) with psoriatic arthritis, based on body weight, is shown below (Table 2).
| Body Weight of Patient at the Time of Dosing | Recommended Dose |
|---|---|
| 60 kg or more | 45 mg |
| greater than 100 kg with co-existent moderate-to-severe plaque psoriasis | 90 mg |
There is no dosage form for STEQEYMA that allows weight-based dosing for pediatric patients below 60 kg.
Recommended Dosage in Crohn's Disease and Ulcerative Colitis
Intravenous Induction Adult Dosage Regimen
A single intravenous infusion dose of STEQEYMA using the weight-based dosage regimen specified in Table 3.
| Body Weight of Patient at the time of dosing | Dose | Number of 130 mg/26 mL (5 mg/mL) STEQEYMA vials |
|---|---|---|
| 55 kg or less | 260 mg | 2 |
| more than 55 kg to 85 kg | 390 mg | 3 |
| more than 85 kg | 520 mg | 4 |
General Considerations for Administration
- STEQEYMA is intended for use under the guidance and supervision of a healthcare provider. STEQEYMA should only be administered to patients who will be closely monitored and have regular follow-up visits with a healthcare provider. The appropriate dose should be determined by a healthcare provider using the patient's current weight at the time of dosing. In pediatric patients, it is recommended that STEQEYMA be administered by a healthcare provider. If a healthcare provider determines that it is appropriate, a patient may self-inject or a caregiver may inject STEQEYMA after proper training in subcutaneous injection technique. Instruct patients to follow the directions provided in the Medication Guide.
- It is recommended that each injection be administered at a different anatomic location (such as upper arms, gluteal regions, thighs, or any quadrant of abdomen) than the previous injection, and not into areas where the skin is tender, bruised, erythematous, or indurated.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Prior to administration, visually inspect STEQEYMA for particulate matter and discoloration. STEQEYMA is a colorless to pale yellow solution and may contain a few small translucent or white particles. Do not use STEQEYMA if it is discolored or cloudy, or if other particulate matter is present. STEQEYMA does not contain preservatives; therefore, discard any unused product remaining in the vial and/or syringe.
Instructions for Administration of STEQEYMA Prefilled Syringes Equipped with Needle Safety Guard
Refer to the diagram below for the provided instructions.

- Remove the needle cap when you are ready to inject STEQEYMA by holding the body of the prefilled syringe in one hand between the thumb and index fingers. Do not hold the plunger while removing the cap. Do not use the prefilled syringe if it has been dropped without the needle cover in place.
- Inject STEQEYMA subcutaneously as recommended.
- Inject all of the liquid by using your thumb to push the plunger all the way down. If the plunger is not fully pressed, the needle guard will not extend to cover the needle when it is removed.
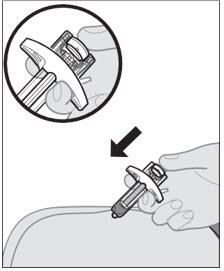
- After the prefilled syringe is empty, slowly lift your thumb from the plunger rod until the needle is completely covered by the needle guard, as shown by the illustration below:
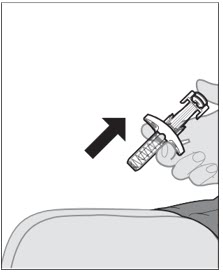
- Used syringes should be placed in a puncture-resistant container.
Preparation and Administration of STEQEYMA 130 mg/26 mL (5 mg/mL) Vial for Intravenous Infusion (Crohn's Disease and Ulcerative Colitis)
STEQEYMA solution for intravenous infusion must be diluted, prepared, and infused by a healthcare professional using aseptic technique.
- 1.
- Calculate the dose and the number of STEQEYMA vials needed based on patient weight (Table 3). Each 26 mL vial of STEQEYMA contains 130 mg of ustekinumab-stba.
- 2.
- Withdraw, and then discard a volume of the 0.9% Sodium Chloride Injection, USP from the 250 mL infusion bag equal to the volume of STEQEYMA to be added (discard 26 mL sodium chloride for each vial of STEQEYMA needed, for 2 vials- discard 52 mL, for 3 vials- discard 78 mL, 4 vials- discard 104 mL). Alternatively, a 250 mL infusion bag containing 0.45% Sodium Chloride Injection, USP may be used.
- 3.
- Withdraw 26 mL of STEQEYMA from each vial needed and add it to the 250 mL infusion bag. The final volume in the infusion bag should be 250 mL. Gently mix.
- 4.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use if visibly opaque particles, discoloration or foreign particles are observed.
- 5.
- Infuse the diluted solution over a period of at least one hour. Once diluted, the infusion should be completely administered within four hours of the dilution in the infusion bag.
- 6.
- Use only an infusion set with an in-line, sterile, non-pyrogenic, low protein-binding filter (pore size 0.2 micrometer).
- 7.
- Do not infuse STEQEYMA concomitantly in the same intravenous line with other agents.
- 8.
- STEQEYMA does not contain preservatives. Each vial is for one-time use in only one patient. Discard any remaining solution. Dispose any unused medicinal product in accordance with local requirements.
Storage
If necessary, the diluted infusion solution may be kept at room temperature up to 30°C (86°F) for up to 3 hours. Storage time at room temperature begins once the diluted solution has been prepared. The infusion should be completed within 4 hours after the dilution in the infusion bag (cumulative time after preparation including the storage and the infusion period). Do not freeze. Discard any unused portion of the infusion solution.
Frequently asked questions
- What biosimilars have been approved in the United States?
- Do I qualify for the Stelara copay card & how can I save?
- What is the mechanism of action of Stelara and how does it work?
- What are the new drugs for plaque psoriasis?
- How long can you keep Stelara in or out of the fridge?
- How quickly or how long before Stelara starts to work?
- Can you get a flu shot or take antibiotics while on Stelara?
- How is Stelara injected or administered?
More about Steqeyma (ustekinumab)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Drug images
- Side effects
- During pregnancy
- FDA approval history
- Drug class: interleukin inhibitors
- Breastfeeding
- En español
Patient resources
Other brands
Stelara, Yesintek, Wezlana, Selarsdi, ... +3 more
Professional resources
Other brands
Stelara, Yesintek, Wezlana, Selarsdi, ... +3 more
Related treatment guides
See also:
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.