Botox Cosmetic Dosage
Generic name: BOTULINUM TOXIN TYPE A 50[USP'U]
Dosage form: injection, powder, lyophilized, for solution
Drug class: Skeletal muscle relaxants
Medically reviewed by Drugs.com. Last updated on Oct 18, 2024.
2.1 Instructions for Safe Use
The potency Units of BOTOX Cosmetic (onabotulinumtoxinA) for injection are specific to the preparation and assay method utilized. BOTOX Cosmetic is not equivalent to other preparations of botulinum toxin products, and therefore, units of biological activity of BOTOX Cosmetic cannot be compared to nor converted into units of any other botulinum toxin products assessed with any other specific assay method.
Follow indication specific dosage and administration recommendations. Do not exceed the maximum recommended cumulative dose in a treatment session for any indication.
The safe and effective use of BOTOX Cosmetic depends upon proper storage of the product, selection of the correct dose, and proper reconstitution and administration techniques. Physicians administering BOTOX Cosmetic must understand the relevant neuromuscular and structural anatomy of the area involved and any alterations to the anatomy due to prior surgical procedures and disease.
Do not use BOTOX Cosmetic and contact AbbVie (1-800-678-1605) if:
- The tamper evident features on the carton appear to be broken or compromised, or
- The U.S. License number 1889 is not present on the vial label and carton labeling
2.2 Recommended Dose
The total recommended dose in adult patients by treatment area is shown in Table 1. BOTOX Cosmetic is administered by intramuscular injection.
| Treatment Area | Total Recommended Treatment Dose |
| Glabellar lines | 20 Units in 0.5 mL |
| Lateral canthal lines | 24 Units in 0.6 mL |
| Forehead lines and glabellar lines | 40 Units in 1 mL |
| Platysma bands | One band on each side: 26 Units in 0.65 mL 1 band on one side, 2 bands on the other side: 31 Units in 0.78 mL Two bands on each side: 36 Units in 0.9 mL |
The safety and effectiveness of dosing with BOTOX Cosmetic more frequently than every 3 months have not been clinically evaluated.
2.3 Preparation and Reconstitution Instructions
BOTOX Cosmetic is supplied in single-dose 50 Units and 100 Units per vial. Prior to intramuscular injection, reconstitute each vacuum-dried vial of BOTOX Cosmetic with sterile, preservative-free 0.9% Sodium Chloride Injection USP (see Table 2). Draw up the proper amount of diluent in the appropriate size needle and syringe to obtain a reconstituted solution at a concentration of 4 Units/0.1 mL.
| Vial | Amount of Diluent* Added | Resulting Dose Units per 0.1 mL |
| 50 Units | 1.25 mL | 4 Units |
| 100 Units | 2.5 mL | 4 Units |
*Preservative-free 0.9% Sodium Chloride Injection, USP Only
Slowly inject the diluent into the vial. Discard the vial if a vacuum does not pull the diluent into the vial. Gently mix BOTOX Cosmetic with the saline by rotating the vial. Record the date and time of reconstitution on the space on the label. Administer BOTOX Cosmetic within 24 hours after reconstitution. During this time period, store reconstituted BOTOX Cosmetic in a refrigerator at 2°C to 8°C (36°F to 46°F). Do not freeze reconstituted BOTOX Cosmetic. BOTOX Cosmetic vials are for single-dose only. Discard any remaining solution.
Reconstituted BOTOX Cosmetic is clear, colorless to slightly yellow solution, and free of particulate matter. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration and whenever the solution and the container permit. Do not use if the solution is cloudy or contains flakes or particles.
2.4 Administration
Draw properly reconstituted toxin into the sterile syringe, preferably a tuberculin syringe, and expel any air bubbles in the syringe barrel (see Table 3). Remove the needle used to reconstitute the product and attach a 30-33 gauge needle. Confirm the patency of the needle.
| Treatment Area | Amount of Reconstituted Toxin to Draw |
| Glabellar lines | At least 0.5 mL |
| Lateral canthal lines | At least 0.6 mL |
| Forehead lines and glabellar lines | At least 1 mL |
| Platysma bands |
|
Glabellar Lines
An effective dose for facial lines is determined by gross observation of the patient’s ability to activate the superficial muscles injected.
In order to reduce the complication of ptosis, take the following steps:
- Avoid injection near the levator palpebrae superioris, particularly in patients with larger brow depressor complexes.
- Place lateral corrugator injections at least 1 cm above the bony supraorbital ridge.
- Ensure the injected volume/dose is accurate and where feasible kept to a minimum.
- Do not inject toxin closer than 1 cm above the central eyebrow.
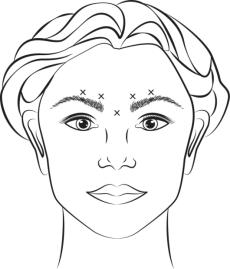
Inject 4 Units (0.1 mL) of reconstituted BOTOX Cosmetic intramuscularly into each of 5 sites, 2 in each corrugator muscle and 1 in the procerus muscle for a total dose of 20 Units (see Figure 1). Typically, the initial doses of reconstituted BOTOX Cosmetic induce chemical denervation of the injected muscles one to two days after injection, increasing in intensity during the first week.
The duration of effect of BOTOX Cosmetic for glabellar lines is approximately 3-4 months.
Figure 1:
Lateral Canthal Lines
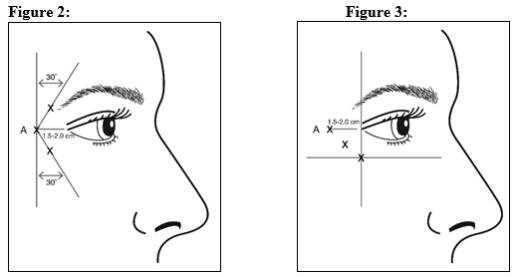
Give injections with the needle bevel tip up and oriented away from the eye. Inject 4 Units (0.1 mL) of reconstituted BOTOX Cosmetic into 3 sites per side (6 total injection points) in the lateral orbicularis oculi muscle for a total of 24 Units (0.6 mL) (12 Units per side). Administer the first injection (A) approximately 1.5-2.0 cm temporal to the lateral canthus and just temporal to the orbital rim. If the lines in the lateral canthal region are above and below the lateral canthus, inject per Figure 2. Alternatively, if the lines in the lateral canthal region are primarily below the lateral canthus, inject per Figure 3.
For simultaneous treatment with glabellar lines, the dose is 24 Units for lateral canthal lines and 20 Units for glabellar lines (see Glabellar Lines Administration and Figure 1), with a total dose of 44 Units.
Forehead Lines in Conjunction with Glabellar Lines
Treat forehead lines in conjunction with glabellar lines (see Glabellar Lines Administration and Figure 1) to minimize the potential for brow ptosis. The recommended total dose for treatment of forehead lines (20 Units [0.5 mL]) in conjunction with glabellar lines (20 Units [0.5 mL]) is 40 Units (1 mL).
When identifying the location of the appropriate injection sites in the frontalis muscle, assess the overall relationship between the size of the subject’s forehead, and the distribution of frontalis muscle activity.
Locate the following horizontal treatment rows by light palpation of the forehead at rest and maximum eyebrow elevation:
- Superior Margin of Frontalis Activity: approximately 1 cm above the most superior forehead crease
- Lower Treatment Row: midway between the superior margin of frontalis activity and the eyebrow, at least 2 cm above the eyebrow
- Upper Treatment Row: midway between the superior margin of frontalis activity and lower treatment row
Inject 4 Units (0.1 mL) of reconstituted BOTOX Cosmetic into 5 sites in the frontalis muscle, for a total of 20 Units (0.5 mL). Place the 5 injections at the intersection of the horizontal treatment rows with the following vertical landmarks (see Figure 4):
- On the lower treatment row at the midline of the face, and 0.5 – 1.5 cm medial to the palpated temporal fusion line (temporal crest); repeat for the other side.
- On the upper treatment row, midway between the lateral and medial sites on the lower treatment row; repeat for the other side.
Figure 4:

For simultaneous treatment with lateral canthal lines, the total dose is 64 Units, comprised of 20 Units for forehead lines, 20 Units for glabellar lines, and 24 Units for lateral canthal lines (see Lateral Canthal Lines Administration and Figures 2 and 3).
Platysma Bands
Using an appropriately sized sterile syringe, needle, and aseptic technique, inject 2 Units (0.05 mL) of reconstituted BOTOX Cosmetic into 4 sites in the upper segment of platysma muscle, below the jawline on each side. For each side, administer the 4 jawline injections to the upper platysma muscle approximately 1 to 2 cm inferior and parallel to the lower mandibular border. Ensure the anterior injection site is in line with the oral commissure, and the posterior injection is slightly anterior to the angle of the mandible. Administer the remaining 2 injections equidistant (approximately 1 to 2 cm apart) between the anterior and posterior injection points (see Figures 5 and 6).
In addition, inject 1 Unit (0.025 mL) of reconstituted BOTOX Cosmetic into 5 sites along each vertical neck band, 1 to 2 vertical neck bands per side. For each vertical neck band identified, 1 to 2 per side, distribute 5 injections vertically approximately 1 to 2 cm apart (see Figures 5 and 6). Ensure the most superior injection site is approximately 1 to 2 cm inferior to the jawline injections.
Depending on platysma band severity, the total dose may be 26 Units (1 band/side), 31 Units (1 band one side, 2 bands other side), or 36 Units (2 bands/side) (see Table 4 and Figures 5 and 6 below).
Figure 5: Injection Sites for Platysma Band (2 Bands) |
Figure 6: Injection Sites for Platysma Band (1 Band) |
Administer all platysma muscle injections superficially and intramuscularly with the needle perpendicular to the surface of the skin. For vertical neck band injections, identify each band while the patient is contracting their platysma. Gently pinch the band to isolate the muscle from nearby anatomical structures during administration (see Table 4).
To reduce injection-related complications, administer injection at least 1 cm inferior to the lower mandibular border. Do not inject into structures deep to the platysma muscle, particularly in the anterior region of the neck.
| Platysma Bands Presentation | Jawline Injections | Neck Band Injections | Total Number of Injection Sites | Total Dose |
| 1 band on each side | 2 Units (0.05 mL) into 4 sites per side (total of 8 sites and 16 Units) | 1 Unit (0.025 mL) into 5 sites per band (total of 10 sites and 10 Units) | 18 sites | 26 Units (0.65 mL) |
| 1 band on one side, 2 bands on the other side |
2 Units (0.05 mL) into 4 sites per side (total of 8 sites and 16 Units) | 1 Unit (0.025 mL) into 5 sites per band (total of 15 sites and 15 Units) | 23 sites | 31 Units (0.78 mL) |
| 2 bands on each side | 2 Units (0.05 mL) into 4 sites per side (total of 8 sites and 16 Units) | 1 Unit (0.025 mL) into 5 sites per band (total of 20 sites and 20 Units) | 28 sites | 36 Units (0.9 mL) |
Frequently asked questions
- Masseter Botox for Jaw Slimming: Benefits, Cost, Side Effects & Results
- Botox Vs Botox Cosmetic: What is the difference?
- What is botulinum toxin used to treat?
- Dysport vs Botox: Key Differences and Effectiveness
More about Botox Cosmetic (onabotulinumtoxinA)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (12)
- Drug images
- Latest FDA alerts (3)
- Side effects
- During pregnancy
- FDA approval history
- Drug class: skeletal muscle relaxants
- Breastfeeding
- En español
Patient resources
Professional resources
Other formulations
Related treatment guides
See also:
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.