Zeniquin Tablets (25 mg) (Canada)
This treatment applies to the following species: Company: Zoetis
Company: Zoetis
marbofloxacin tablets
Veterinary Use Only
Antibacterial for oral use in dogs and cats
DIN 02241415
DIN 02241414
DIN 02241413
DIN 02241412
Description
Marbofloxacin is a synthetic broad-spectrum antibacterial agent from the fluoroquinolone class of chemotherapeutic agents. Marbofloxacin is the non-proprietary designation for 9-fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido[3,2,1-ij][4,1,2] benzoxadiazine-6-carboxylic acid. The empirical formula is C17H19FN4O4 and the molecular weight is 362.36.
Figure 1: Chemical structure of marbofloxacin.
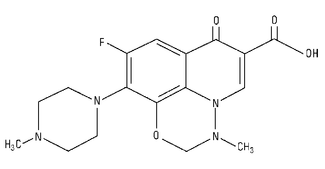
Zeniquin tablets contain marbofloxacin as the medicinal ingredient at 25 mg, 50 mg, 100 mg, or 200 mg per tablet.
Zeniquin Tablets (25 mg) Indications
Zeniquin (marbofloxacin) tablets are indicated for the treatment of infections associated with bacteria susceptible to marbofloxacin, in dogs and cats.
EFFICACY CONFIRMATION: Clinical efficacy was established in skin and soft tissue infections (superficial pyodermas, wounds and abscesses) associated with susceptible strains of Staphylococcus intermedius in dogs and urinary tract infections (cystitis) associated with susceptible strains of Escherichia coli and Proteus mirabilis in dogs. Clinical efficacy was established in skin and soft tissue infections (wounds and abscesses) associated with susceptible strains of Pasteurella multocida in the cat. Other bacterial pathogens isolated in clinical field studies are provided in the Microbiology section.
Dosage and Administration
For the treatment of skin and soft tissue infections in dogs and cats, Zeniquin tablets should be given at a dosage of 2.75 mg/kg once daily for 5 to 14 days.For the treatment of urinary tract infections in dogs, Zeniquin tablets should be given at a dosage of 2.75 mg/kg once daily for 10 to 14 days.
Efficacy for the treatment of skin and soft tissue infections was confirmed at a dosage of 2.75 mg/kg. In dogs, the dosage may be safely increased to 5.5 mg/kg for two to three days beyond the cessation of clinical signs for a maximum of 30 days, should the patient require more intensive antimicrobial therapy. Some factors to be considered in the determination of dosage are the nature and severity of the infection, the susceptibility of the pathogen, and ability of the patient to combat infection. In dogs and cats, if no improvement is noted within 5 days, the diagnosis should be re-evaluated and a different course of therapy considered. Clinical studies did not reveal improved clinical resolution rates at 5.5 mg/kg in comparison to a dosage of 2.75 mg/kg.
Clinical Pharmacology
Marbofloxacin is rapidly and almost completely absorbed from the gastrointestinal tract following oral administration to fasted animals. Bioavailability following oral dosing was 96% in studies utilizing the same animals. The effects of concomitant feeding on the absorption of marbofloxacin have not been determined. Divalent cations are generally known to diminish the absorption of fluoroquinolones. (See Drug Interactions.) In vitro protein binding of marbofloxacin in the dog was 9.1% and was 7.3% in cats. In the dog, approximately 40% of an oral dose of marbofloxacin is excreted unchanged in the urine. Excretion in the feces, also as unchanged drug, is the other major route of elimination in dogs. Ten to 15% of marbofloxacin is metabolized by the liver in dogs. In the cat, approximately 70% of an oral dose is excreted in the urine as marbofloxacin and metabolites.Pharmacokinetic parameters related to intravenous dosing were estimated in a study of six healthy adult beagle dogs, and are summarized in Table 1.
Table 1: Mean pharmacokinetic parameters following intravenous administration of marbofloxacin to six adult beagle dogs at a dosage of 5.5 mg/kg.
|
Parameter |
Estimate |
SEM1 |
|
Total body clearance, (mL/h•kg) |
96 |
4 |
|
Volume of distribution at steady state, Vss, (L/kg) |
1.19 |
0.02 |
|
AUC0-inf (µg•h/mL) |
58 |
2 |
|
Terminal plasma elimination half-life, t1/2 (h) |
9.24 |
0.65 |
1 SEM = standard error of the mean
Marbofloxacin plasma concentrations were determined over time in healthy adult beagle dogs (6 dogs per dosage group) following single oral doses of 2.75 mg/kg or 5.5 mg/kg. Absorption following oral administration increased in dose proportion to the dosage without effect on elimination rate. Marbofloxacin plasma concentrations were determined over time in 7 healthy adult male cats following a single oral dose of 5.5 mg/kg. Plasma pharmacokinetic parameters for dogs and cats are summarized in Figures 2 and 3 and in Table 2 below.
Figure 2: Mean plasma concentrations (µg/mL) following single oral administration of marbofloxacin to adult beagle dogs at dosages of 2.75 mg/kg or 5.5 mg/kg.
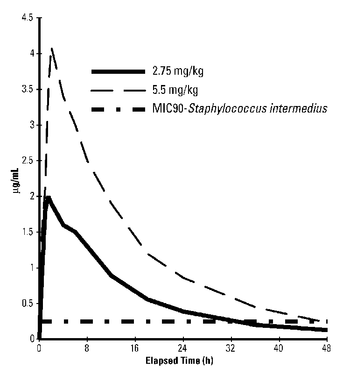
Figure 3: Mean plasma concentrations (µg/mL) following single oral administration of marbofloxacin to adult cats at a dosage of 5.5 mg/kg.
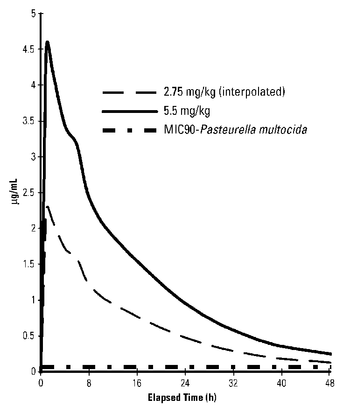
Table 2: Mean pharmacokinetic parameters following oral administration of marbofloxacin tablets to adult beagle dogs at a dosage of 2.75 mg/kg or 5.5 mg/kg and to cats at 5.5 mg/kg.
|
Parameter |
Dog Estimate |
Dog Estimate |
SE2 |
Cat Estimate |
SE2 |
|
Time of maximum concentration, TMAX, (h) |
1.5 |
1.8 |
0.1 |
1.2 |
0.2 |
|
Maximum concentration, Cmax, (µg/mL) |
2.0 |
4.2 |
0.2 |
4.8 |
0.3 |
|
AUC0-inf (µg•h/mL) |
31 |
64 |
2 |
70 |
2.0 |
|
Terminal plasma elimination half-life, t1/2 (h) |
11 |
11 |
0 |
13 |
0 |
2 SE = standard error
Marbofloxacin is widely distributed in body tissues. Tissue concentrations of marbofloxacin were determined in healthy male beagle dogs (4 dogs per time period) at 2, 18 and 24 hours after dosing and are summarized in Table 3. Concentrations of drug equal to or greater than the MIC for predominant pathogens are reached in tissues by 2 hours after dosing and are maintained throughout the interval between doses.
Table 3: Tissue distribution following a single oral administration of marbofloxacin tablets to adult beagle dogs.
|
Tissue |
Marbofloxacin Concentration (µg/g) |
|||||
|
2 hours |
18 hours |
24 hours |
||||
|
2.75 mg/kg |
5.5 mg/kg |
2.75 mg/kg |
5.5 mg/kg |
2.75 mg/kg |
5.5 mg/kg |
|
|
bladder |
4.8 |
12 |
2.6 |
6.0 |
1.1 |
1.8 |
|
bone marrow |
3.1 |
4.6 |
1.5 |
1.3 |
0.73 |
0.92 |
|
feces |
15 |
18 |
48 |
52 |
26 |
47 |
|
jejunum |
3.6 |
7.8 |
1.3 |
2.0 |
0.74 |
1.1 |
|
kidney |
7.1 |
13 |
1.4 |
2.7 |
0.95 |
1.6 |
|
lung |
3.0 |
5.5 |
0.81 |
1.5 |
0.57 |
1.0 |
|
lymph node |
5.5 |
8.3 |
1.3 |
2.3 |
1.0 |
2.0 |
|
muscle |
4.1 |
7.5 |
0.98 |
1.8 |
0.72 |
1.2 |
|
prostate |
5.6 |
11 |
1.8 |
2.7 |
1.1 |
2.0 |
|
skin |
1.9 |
3.2 |
0.41 |
0.71 |
0.32 |
0.46 |
MICROBIOLOGY: The primary action of fluoroquinolones is to inhibit the bacterial enzyme, DNA gyrase. In susceptible organisms, fluoroquinolones are rapidly bactericidal at relatively low concentrations Marbofloxacin is bactericidal against a broad range of aerobic Gram negative and Gram positive organisms. Minimum bactericidal concentrations are typically the same as, or one doubling dilution higher than, the minimum inhibitory concentrations (MICs). MICs of aerobic bacteria isolated in clinical field studies performed in the United States between 1994 and 1998 were determined using National Committee for Clinical Laboratory Standards (NCCLS) standards, and are shown in Tables 4 and 5.
Table 4: MIC Values3 (µg/mL) of marbofloxacin against bacteria isolated from skin, soft tissue and urinary tract infections in dogs enrolled in clinical studies conducted during 1994-1996.
|
Organism |
Number of Isolates |
MIC50 |
MIC90 |
MIC Range |
|
Staphylococcus intermedius |
135 |
0.25 |
0.25 |
0.125-2 |
|
Escherichia coli |
61 |
0.03 |
0.06 |
0.015-2 |
|
Proteus mirabilis |
35 |
0.06 |
0.125 |
0.03-0.25 |
|
Beta-hemolytic Streptococcus, (not Group A or Group B) |
25 |
1 |
2 |
0.5-16 |
|
Streptococcus, Group D enterococcus |
16 |
1 |
4 |
0.008-4 |
|
Pasteurella multocida |
13 |
0.015 |
0.06 |
£0.008-0.5 |
|
Staphylococcus aureus |
12 |
0.25 |
0.25 |
0.25-0.5 |
|
Enterococcus faecalis |
11 |
2 |
2 |
1-4 |
|
Klebsiella pneumoniae |
11 |
0.06 |
0.06 |
0.01-0.06 |
|
Pseudomonas species |
9 |
** |
** |
0.06-1 |
|
Pseudomonas aeruginosa |
7 |
** |
** |
0.25-1 |
3 The correlation between in vitro susceptibility data (MIC) and clinical response has not been determined.
** MIC50 and MIC90 not calculated due to insufficient number of isolates.
Table 5: MIC Values4 (µg/mL) of marbofloxacin against bacteria isolated from skin and soft tissue infections in cats enrolled in clinical studies conducted in 1995 and 1998.
|
Organism |
Number of Isolates |
MIC50 |
MIC90 |
MIC Range |
|
Pasteurella multocida |
135 |
0.03 |
0.06 |
£0.008-0.25 |
|
Beta-hemolytic Streptococcus |
22 |
1 |
1 |
0.06-1 |
|
Staphylococcus aureus |
21 |
0.25 |
0.5 |
0.125-1 |
|
Corynebacterium spp. |
14 |
0.5 |
1 |
0.25-2 |
|
Staphylococcus intermedius |
11 |
0.25 |
0.5 |
0.03-0.5 |
|
Enterococcus faecalis |
10 |
2.0 |
2.0 |
1.0-2.0 |
|
Escherichia coli |
10 |
0.03 |
0.03 |
0.015-0.03 |
|
Bacillus spp. |
10 |
0.25 |
0.25 |
0.125-0.25 |
4The correlation between in vitro susceptibility data (MIC) and clinical response has not been determined.
DRUG INTERACTIONS: Compounds (e.g., sucralfate, antacids, and mineral supplements) containing divalent and trivalent cations (e.g., iron, aluminum, calcium, magnesium, and zinc) can interfere with the absorption of quinolones which may result in a decrease in product bioavailability.
Contraindications
Marbofloxacin and other quinolones have been shown to cause arthropathy in immature animals of most species tested, the dog being particularly sensitive to this side effect. Marbofloxacin is contraindicated in immature dogs during the rapid growth phase (small and medium breeds up to 8 months of age, large breeds up to 12 months of age and giant breeds up to 18 months of age). Marbofloxacin is also contraindicated in cats under 12 months of age. Marbofloxacin is contraindicated in dogs and cats known to be hypersensitive to quinolones.
CAUTIONS: Quinolones should be used with caution in animals with known or suspected central nervous system (CNS) disorders. In such animals, quinolones have, in rare instances, been associated with CNS stimulation which may lead to convulsive seizures. Quinolones have been shown to produce erosions of cartilage of weight-bearing joints and other signs of arthropathy in immature animals of various species. The use of fluoroquinolones in cats has been reported to adversely affect the retina and such products should be used with caution in cats.
Safety in breeding, pregnant or lactating dogs and cats has not been established.
Warnings
To limit the potential development of antimicrobial resistance: - Fluoroquinolone drugs such as Zeniquin should not be used indiscriminately - Zeniquin should not be used in food producing animals.Keep out of reach of children.
Safety
Dogs: The toxicity of marbofloxacin was assessed in 12 to 14 month old beagle dogs administered marbofloxacin at 5.5, 16.5 and 27.5 mg/kg/day for 42 days. These dosages represent 2, 6, and 10 times the recommended dosage of 2.75 mg/kg/day. Vomiting, reddened skin (usually involving the ears) and reddened mucous membranes were occasionally observed in all groups, including controls, but were noted most frequently in the 10x group. Decreased food consumption and weight loss were significant in the 6x and 10x groups. No clinical lameness was noted in any of the treated animals. No gross or histopathologic lesions were detected in the joints of animals given 5.5 or 16.5 mg/kg. Alterations in articular cartilage were observed grossly in three of the 10x group dogs and in one placebo dog. Cartilage lesions in treated dogs were similar to those in control dogs, but were not typical of those produced by fluoroquinolones.
Marbofloxacin was administered to 12 to 14 month old beagle dogs at a dose of 55 mg/kg/day (20 times the recommended dosage) for 12 days. Decreased food consumption, vomiting, dehydration, excessive salivation, tremors, reddened skin, facial swelling, decreased activity and weight loss were seen in treated dogs. No clinical lameness or histopathologic lesions of joints were noted.
Marbofloxacin administered to 3 to 4 month old large breed purpose-bred mongrel dogs at a dosage of 11 mg/kg/day (four times the recommended dosage) for 14 days resulted in marked lameness in all dogs due to articular cartilage lesions. Lameness was accompanied by decreased appetite and activity.
Cats: Marbofloxacin was administered for 42 consecutive days to 24 cats approximately eight months old (eight cats per treatment group) at the dosages of 5.5, 16.5 and 27.5 mg/kg/day. These dosages represent 2, 6, and 10 times the recommended dosage of 2.75 mg/kg/day. Treatment with marbofloxacin did not produce adverse effects on body weights, food consumption, ophthalmology, serum chemistry, urinalysis or organ weight parameters. A significant decrease in the neutrophil count occurred in some cats in all treatment groups and in some cases the absolute neutrophil count was below normal reference values. Clinical signs were occasionally noted in cats in the highest dosage group: excessive salivation in 4/8 cats and redness of ear pinnae in 2/8 cats. Macroscopic changes in the articular cartilage of femurs were seen in one cat receiving 16.5 mg/kg and in three cats receiving 27.5 mg/kg. Microscopic changes typical of those associated with fluoroquinolone toxicity were present in one cat receiving 5.5 mg/kg and one cat receiving 27.5mg/kg; these cats also had closure of the epiphyseal growth plates. There was no evidence of lameness during the course of the study. A perivascular to diffuse dermatitis was seen microscopically in one mid-dose cat and four high-dose cats.
Marbofloxacin was also administered orally to six cats approximately eight months of age for 14 consecutive days at a dosage of 55 mg/kg/day (20 times the recommended dosage). Clinical signs associated with drug intolerance were excessive salivation in 5/6 cats and redness of ear pinnae in all cats after 8 days of treatment. Decreased food intake was noted in some animals, primarily males, when compared to controls. Perivascular to diffuse dermatitis was seen microscopically in the pinnae of all treated animals and in the standard skin samples of several animals. There was focal or multifocal articular chondropathy typical of fluoroquinolone toxicity in two of the six treated animals. One treated cat had a duodenal mucosal erosion and one treated cat had a pyloric ulcer. There were no observations of lameness and no adverse effects on ophthalmology, hematology, clinical chemistry, urinalysis, or organ weight parameters.
A study was conducted to investigate the effect of marbofloxacin on articular cartilage of skeletally mature cats 12 to 14 months of age. Forty cats were randomly assigned to four groups of ten cats each. Groups received placebo or marbofloxacin at dosages 2.75, 8.25 or 16.5 mg/kg/day (1,3, and 6 times the recommended dosage) for 42 consecutive days. There were no treatment-related pathological changes in the joints or other tissues. Emesis and soft stools was noted in all treatment groups, including placebo, and increased in frequency with increasing dose and duration of treatment. Emesis was more apparent in the high-dose males.
Adverse Reactions
The following clinical signs were reported during the course of clinical field studies in dogs receiving marbofloxacin at dosages up to 5.5 mg/kg daily: decreased or loss of appetite (5.4%), decreased activity (4.4%), and vomiting (2.9%). The following signs were reported in less than one percent of cases: increased thirst, soft stool/diarrhea, behavioral changes, shivering/shaking/tremors, and ataxia. One dog which had a seizure the day before study enrollment experienced a seizure while on marbofloxacin therapy.The following clinical signs were reported during clinical field studies in cats receiving dosages up to 5.5 mg/kg daily: diarrhea (2.1%) and soft stool (1.4%). Vomiting was reported in less than one percent of cases in cats.
Storage
Store between 15 and 30°C.PRESENTATION: Zeniquin marbofloxacin scored coated tablets are supplied as described below:
|
Concentration |
No. of tablets per bottle |
|
25 mg |
100 & 250 |
|
50 mg |
100 |
|
100 mg |
50 |
|
200 mg |
50 |
Zoetis is a trademark and Zeniquin is a registered trademark of Zoetis or its licensors, used under license by Zoetis Canada Inc.
Zoetis Canada Inc., Kirkland QC H9H 4M7
8485-11-2
30434000
CPN: 1198126.7
16,740 TRANS-CANADA HIGHWAY, KIRKLAND, QC, H9H 4M7
| Order Desk: | 800-663-8888 | |
| Technical Services Canada: | 800-461-0917 | |
| Technical Services USA: | 800-366-5288 | |
| Website: | www.zoetis.ca |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-08-27
