Sodium Bicarbonate 8.4%
This treatment applies to the following species: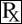 Company: Neogen
Company: Neogen
STERILE SOLUTION
Sodium Bicarbonate 8.4% Caution
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
FOR ANIMAL USE ONLY
KEEP OUT OF REACH OF CHILDREN
Each mL contains:
|
Sodium Bicarbonate |
84 mg (equal to 1 mEq/mL) |
|
Water for Injection |
q.s. |
Sodium Bicarbonate 8.4% Indications
For treatment of metabolic acidosis. See package insert for specific indications.
Dosage and Administration
Administer intravenously. See package insert for complete directions for use and dosage information.
Store at a temperature between 15° and 30°C (59° - 86°F).
Avoid freezing.
Warning
This is a sterile single dose vial. No preservatives have been added. Discard unused portion after use.
Do not use if solution is hazy, cloudy, or contains a precipitate.
The total osmolar concentration of this product is approximately 2000 mOsm/L.
Read entire package insert before use.
NEOGEN® Vet
Manufactured by: Nova-Tech, Grand Island, NE 68801
Manufactured for: Neogen Corporation, Lexington, KY 40511
859-254-1221
animalsafety.neogen.com
|
Net Contents: |
NDC: |
Item No. |
|
|
100 mL |
59051-9078-5 |
09078 |
RMS 92-305 L568-0518 |
CPN: 1491012.8
944 NANDINO BLVD, LEXINGTON, KY, 40511-1205
| Telephone: | 859-254-1221 | |
| Order Desk: | 800-525-2022 | |
| Fax: | 859-255-5532 | |
| Website: | http://animalsafety.neogen.com | |
| Email: | inform@neogen.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-08-27
