Simparica chewable tablets (120 mg) (Canada)
This treatment applies to the following species: Company: Zoetis
Company: Zoetis
sarolaner chewable tablets
DIN 02452014, DIN 02452022
DIN 02452030, DIN 02452049
DIN 02452057, DIN 02452065
Veterinary Use Only
Parasiticide for dogs
Description
SIMPARICA® (sarolaner) is a flavoured chewable tablet containing 5, 10, 20, 40, 80, or 120 mg of sarolaner, an ectoparasiticide intended for administration to dogs according to their weight. Each tablet is formulated to provide a minimum sarolaner dosage of 2 mg/kg (0.91 mg/lb) body weight.
Simparica chewable tablets (120 mg) Indications
SIMPARICA chewable tablets kill ticks and adult fleas, and are indicated for:
● The treatment and control of tick infestations with Ixodes scapularis (black-legged tick), Dermacentor variabilis (American dog tick), Amblyomma americanum (lone star tick), Amblyomma maculatum (Gulf Coast tick), Rhipicephalus sanguineus (brown dog tick) and Haemaphysalis longicornis (Asian longhorned tick) for one month.
● The reduction of Borrelia burgdorferi infection as a direct result of killing adult Ixodes scapularis vector ticks.
● The treatment and prevention of flea infestations (Ctenocephalides felis) for one month.
● The treatment of ear mite infestations caused by Otodectes cynotis.
SIMPARICA chewable tablets are indicated in dogs 6 months of age and older and weighing 1.3 kg (2.8 lbs) or greater.
Dosage and Administration
SIMPARICA chewable tablets are given orally once a month at the recommended dosage of 2-4 mg/kg (0.91-1.82 mg/lb).
Dosage Schedule:
|
Body Weight |
Sarolaner per Tablet (mg) |
Number of Tablets Administered |
|
|
In kg |
In lbs |
||
|
1.3 to 2.5 kg |
2.8 to 5.5 lbs |
5 |
1 |
|
2.6 to 5.0 kg |
>5.5 to 11.0 lbs |
10 |
1 |
|
5.1 to 10.0 kg |
>11.0 to 22.0 lbs |
20 |
1 |
|
10.1 to 20.0 kg |
>22.0 to 44.0 lbs |
40 |
1 |
|
20.1 to 40.0 kg |
>44.0 to 88.0 lbs |
80 |
1 |
|
40.1 to 60.0 kg |
>88.0 to 132.0 lbs |
120 |
1 |
|
60.1 kg and up |
>132.0 lbs and up |
Administer the appropriate combination of tablets |
|
SIMPARICA chewable tablets are flavoured and are readily consumed by dogs when offered by the owner. In a well-controlled US field study, which included 884 doses administered, 59.5% of dogs voluntarily consumed SIMPARICA chewable tablets, an additional 32.01% voluntarily consumed the product when offered with food, and 8.5% were pilled. Re-dosing was required for one dog that voluntarily consumed the product. SIMPARICA chewable tablets may be administered with or without food. Treated animals should be observed for a few minutes to ensure that part of the dose is not lost or refused. If a dose of SIMPARICA chewable tablets is missed, administer that dose immediately and resume a monthly dosing schedule.
SIMPARICA chewable tablets should be administered at monthly intervals. Treatment with SIMPARICA chewable tablets may begin at any time of the year and can continue without interruption.
Due to geographic and climate variations across the country, Canada has a variable and evolving distribution of tick species as well as a variable abundance of flea and tick infestations. A comprehensive plan, based on regional risk assessment and a detailed travel history, is recommended to determine an appropriate dosing plan.
To minimize the likelihood of flea re-infestation, it is important to treat all dogs and cats within a household with an approved flea product.
For the treatment of ear mite infestations, a single dose of SIMPARICA chewable tablets should be administered. A further veterinary examination 30 days after treatment is recommended as some dogs may require a second treatment.
Contraindications
There are no known contraindications for the use of SIMPARICA chewable tablets.
Cautions:
Sarolaner is a member of the isoxazoline class. This class has been associated with neurological adverse reactions including tremors, ataxia, and seizures. Seizures have been reported in dogs receiving isoxazoline class drugs, even in dogs without a history of seizures. Use with caution in dogs with a history of seizures or neurological disorders.
SIMPARICA chewable tablets should not be used in dogs less than 6 months of age (see Animal Safety).
The safe use of SIMPARICA chewable tablets has not been evaluated in breeding, pregnant, or lactating dogs.
Warnings
Keep out of reach of children.
Adverse Reactions
Although all adverse reactions are not reported, the following information is based on voluntary post-approval drug experience reporting. It is generally recognized that this results in significant under-reporting. The adverse events listed here reflect reporting and not necessarily causality. Most reported adverse events, listed below by body system in decreasing order of frequency, were observed very rarely (in less than 1 animal per 10 000 treated):
- Systemic disorders: lack of efficacy, lethargy, anorexia.
- Digestive tract disorders: vomiting, diarrhea.
- Neurological disorders: seizure, tremor, ataxia.
In a well-controlled US field study, which included a total of 479 dogs (315 dogs treated with SIMPARICA chewable tablets and 164 dogs treated with active control once monthly for three treatments), a variety of abnormal clinical signs or potential adverse reactions were reported in both treatment groups.
Clinical signs that occurred at an incidence of ≥2% within the study period are presented in the following table. The most frequently reported adverse events in the sarolaner group were erythema and pruritus; in the active control group, the most frequently reported adverse event was vomiting (emesis).
Percentage of Dogs with Adverse Reactions in the Efficacy Field Study
|
Adverse Reaction |
SIMPARICA chewable tablets (n=315 dogs) |
Active Control |
|
Erythema |
3.5% |
3.0% |
|
Pruritus |
3.5% |
7.3% |
|
Emesis |
3.2% |
9.8% |
|
Ear Discharge |
2.5% |
1.2% |
|
Lethargy |
2.5% |
1.8% |
|
Cough |
2.2% |
3.0% |
Other clinical signs were reported but occurred in <2.0% of dogs. No serious adverse events were attributed to the administration of SIMPARICA chewable tablets.
To report adverse reactions, call Zoetis Canada Inc. at 1-800-461-0917.
Clinical Pharmacology
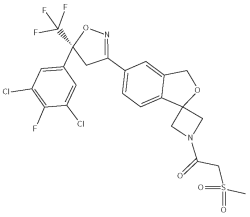
Sarolaner is a member of the isoxazoline class of parasiticides and the chemical name is 1 - (5’ - ((5S) - 5 - (3,5 - Dichloro - 4 - fluorophenyl) - 5 - (trifluoromethyl) - 4,5 - dihydroisoxazol - 3 - yl) - 3’ - H - spiro(azetidine - 3,1’ - (2)benzofuran) - 1 - yl) - 2 - (methylsulfonyl)ethanone. SIMPARICA contains the S-enantiomer of sarolaner.
The chemical structure of the S-enantiomer of sarolaner is:
Mode of Action
Isoxazolines (including sarolaner) are potent inhibitors of the neurotransmitter gamma-aminobutyric acid (GABA) receptor and glutamate receptor function and work at the neuromuscular junction in arthropods. This results in uncontrolled neuromuscular activity leading to rapid death in insects or acarines. Sarolaner has a greater affinity for the GABA receptors in insects compared to mammals.
Pharmacokinetics
Sarolaner is rapidly and well absorbed following oral administration of SIMPARICA chewable tablets. In a study of 12 Beagle dogs, the mean maximum plasma concentration (Cmax) was 1100 ng/mL and the mean time to maximum concentration (Tmax) occurred at 3 hours following a single oral dose of 2 mg/kg to fasted dogs. The mean oral bioavailability was 86% and 107% in fasted and fed dogs, respectively. The mean oral T1/2 value for fasted and fed dogs was 10 and 12 days respectively.
The mean volume of distribution (Vdss) of sarolaner was 2.81 L/kg bodyweight following a 2 mg/kg intravenous dose, indicating that this drug is widely distributed. Sarolaner is highly bound ~99.9%) to plasma proteins. The metabolism of sarolaner appears to be minimal in the dog. The primary route of sarolaner elimination from dogs is biliary excretion with elimination via the feces. Following repeated administration of SIMPARICA chewable tablets once every 28 days for 10 doses to Beagle dogs at 1X, 3X, and 5X the maximum intended clinical dose of 4 mg/kg, steady-state plasma concentrations were reached after the 6th dose.
Efficacy:
Tick Control
In well-controlled laboratory studies, SIMPARICA chewable tablets demonstrated ≥99.1% efficacy 48 hours post-dose against existing infestations of six species of ticks (Ixodes scapularis, Amblyomma americanum, Amblyomma maculatum, Dermacentor variabilis, Rhipicephalus sanguineus, and Haemaphysalis longicornis). Continuing effectiveness ≥94.5% was maintained against weekly re-infestations of all six species for 35 days.
In a laboratory study against A. maculatum, SIMPARICA chewable tablets provided 80.2% reduction in mean live tick counts within 8 hours after the initial treatment, and 99.0% reduction by 12 hours after the initial treatment.
In two separate, well-controlled laboratory studies, SIMPARICA chewable tablets were effective at preventing Borrelia burgdorferi infections after dogs were infested with room temperature, adult I. scapularis vector ticks 28 days post-treatment.
Flea Control
In a well-controlled laboratory study, SIMPARICA chewable tablets showed a significant reduction in adult flea counts 3 hours after initial administration and achieved 100% efficacy at 8 hours post-administration. SIMPARICA chewable tablets continued to demonstrate ≥89.0% efficacy 8 hours after weekly flea re-infestation for 35 days.
In a separate well-controlled laboratory study, SIMPARICA chewable tablets demonstrated 100% efficacy against adult fleas within 24 hours following treatment and maintained 100% efficacy within 24 hours against weekly re-infestations for 35 days.
In a study to simulate a flea-infested home environment, an infestation was established prior to the start of treatment. Dogs were also re-infested with fleas on Days 7, 37 and 67. SIMPARICA chewable tablets administered monthly for three months demonstrated ≥94.3% reduction in adult fleas within 14 days after the first treatment, and reached 100% efficacy from Day 60 onward. In a study to explore flea egg production and viability, SIMPARICA chewable tablets killed fleas before they could lay eggs and thus prevented 100% of flea egg production in an existing infestation and against weekly re-infestations for 35 days.
In a well-controlled 90-day US field study conducted in households with existing flea infestations of varying severity, the efficacy of SIMPARICA chewable tablets against fleas on Day 30, 60 and 90 visits compared to baseline was 99.5%, 99.9% and 100%, respectively. Dogs with signs of flea allergy dermatitis showed improvement in erythema, papules, scaling, alopecia, dermatitis/pyodermatitis and pruritus as a direct result of eliminating fleas from the animals and their environment.
Ear Mite Treatment
In a well-controlled 60-day laboratory study in 8 dogs, a single treatment with SIMPARICA chewable tablets reduced total Otodectes cynotis mite counts by 98.7% within 30 days after treatment and after two monthly doses of SIMPARICA chewable tablets Otodectes cynotis mite counts were reduced to 99.9% on study Day 60.
In a well-controlled 60-day EU field study, SIMPARICA chewable tablets (163 dogs) or a topical combination of moxidectin and imidacloprid (78 dogs) were administered to dogs with naturally acquired ear mite infestation. Efficacy of SIMPARICA chewable tablets was 90.5% after a single administration. The percent efficacy for SIMPARICA chewable tablets was non inferior to the combination of moxidectin and imidacloprid on study Day 30. If live mites were found on Day 30 a second treatment was administered. Of the 15 dogs treated for a second time with SIMPARICA chewable tablets, efficacy was 93.3% after the second administration.
Animal Safety:
In a margin of safety study, SIMPARICA chewable tablets were administered orally to 8-week-old Beagle puppies at doses of 0, 1X, 3X, and 5X the maximum recommended dose (4 mg/kg) at 28-day intervals for 10 doses (8 dogs per group). Dogs in the control group received placebo tablets. No neurological signs were observed in the 1X group. In the 3X group, one male dog exhibited tremors and ataxia post-dose on Day 0; one female dog exhibited tremors on Days 1, 2, 3, and 5; and one female dog exhibited tremors on Day 1. In the 5X group, one female dog had a seizure on Day 61 (5 days after third dose); one female dog had tremors post-dose on Day 0 and abnormal head coordination after dosing on Day 140; and one female dog exhibited seizures associated with the second and fourth doses and tremors associated with the second and third doses. All dogs recovered without treatment. There were no treatment-related neurological signs observed once the dogs reached the age of 6 months, except for the observation of abnormal head coordination in one dog in the 5X group two hours after dosing on Day 140 (dose 6).
In a separate exploratory pharmacokinetic study, one female dog dosed at 12 mg/kg (3X the maximum recommended dose) exhibited lethargy, anorexia, and multiple neurological signs including ataxia, tremors, disorientation, hypersalivation, diminished proprioception, and absent menace, approximately 2 days after a third monthly dose. The first two doses resulted in plasma concentrations that were consistent with those of the other dogs in the treatment group. Starting at 7 hours after the third dose, there was a rapid 2.5 fold increase in plasma concentrations within 41 hours, resulting in a Cmax more than 7-fold higher than the mean Cmax at the maximum recommended use dose. No cause for the sudden increase in sarolaner plasma concentrations was identified.
In a laboratory safety study, sarolaner was safely co-administered at doses up to 20 mg/kg (5X the maximum recommended dose) with moxidectin (up to 30 micrograms/kg) and pyrantel pamoate (up to 50 mg/kg) in avermectin-sensitive collie dogs for a single dose. No adverse signs were observed.
In a well-controlled field study, SIMPARICA chewable tablets were used concurrently with other commonly used medications such as vaccines, anthelmintics, heartworm preventatives, antibiotics, and corticosteroids. No adverse reactions were attributed to the concurrent use of SIMPARICA chewable tablets with other medications.
Storage
Store between 15 and 30°C.
Presentation:
SIMPARICA chewable tablets are available in six flavoured tablet sizes: 5, 10, 20, 40, 80, and 120 mg. Each tablet size is available in color-coded packages of one, three, or six tablets.
Not all pack sizes may be marketed.
Zoetis® and Simparica are registered trademarks of Zoetis or its licensors.
Zoetis Canada Inc., Kirkland QC H9H 4M7
July 2024
CPN: 1198530.8
16,740 TRANS-CANADA HIGHWAY, KIRKLAND, QC, H9H 4M7
| Order Desk: | 800-663-8888 | |
| Technical Services Canada: | 800-461-0917 | |
| Technical Services USA: | 800-366-5288 | |
| Website: | www.zoetis.ca |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-08-27
