RECOMBITEK Oral Bordetella
This treatment applies to the following species:Oral Bordetella SWDO
Dogs
1 mL
Bordetella Bronchiseptica Vaccine
Avirulent Live Culture
RECOMBITEK Oral Bordetella Indications
This product has been shown to be effective for the vaccination of healthy dogs 8 weeks of age or older against canine infectious tracheobronchitis (kennel cough) due to Bordetella bronchiseptica. The duration of immunity is at least 13 months. For more information regarding efficacy and safety data, go to productdata.aphis.usda.gov.
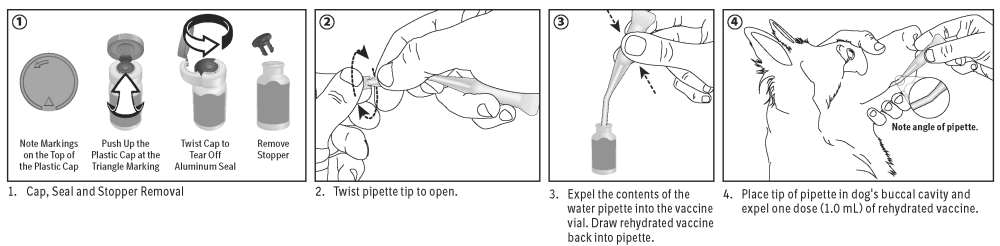
Directions and dosage: Open vaccine vial by removing tear-off cap, seal and stopper. Open water diluent pipette and expel water into the vaccine vial to rehydrate. Shake well. Draw vaccine back into the pipette, place tip of pipette (without needle) in dog’s buccal cavity and expel one dose (1 mL) of rehydrated vaccine. For advice on revaccination frequency, consult your veterinarian.
Precautions
This product is designed for oral use only. Do not vaccinate dogs parenterally. Store out of direct sunlight at 2-8°C (35-46°F). Avoid freezing. Use immediately after reconstitution. Do not mix with other biological products, except as specified on the label. This product has not been tested in pregnant animals. In case of anaphylactoid reaction, administer epinephrine. In case of human exposure, contact a physician. Inactivate unused contents before disposal.For use in animals only. Sold to veterinarians only.
Boehringer Ingelheim Animal Health USA Inc., Athens, GA 30601
Phone: 1 (888) 637-4251
VLN/PCN 124/1081.04
|
Contains: |
|
|
25 doses 25 x 1 Dose, Lyophilized 25 x 1 mL, Sterile Diluent Pipettes |
RM2250R7 |
CPN: 1028371.0
3239 SATELLITE BLVD., BLDG 500, DULUTH, GA, 30096
| Telephone: | 800-325-9167 | |
| Customer Service: | 888-637-4251 | |
| Technical Service: | 888-637-4251 | |
| Fax: | 816-236-2717 | |
| Website: | www.boehringer-ingelheim.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-08-27
