OvuGel (Canada)
This treatment applies to the following species: Company: Vetoquinol
Company: Vetoquinol
TRIPTORELIN INTRAVAGINAL GEL
100 µg/mL (as triptorelin acetate)
VETERINARY USE ONLY
DIN 02424150
Description
OvuGel is a thin, clear to slightly hazy gel. Each mL of OvuGel contains 100 µg of triptorelin (as triptorelin acetate) for intravaginal administration. Triptorelin acetate is a synthetic gonadotropin-releasing hormone (GnRH) agonist which acts at the anterior pituitary gland to induce luteinizing hormone (LH) release, which in turn stimulates ovulation. This physiological response allows for the synchronization of time of insemination in sows following weaning to facilitate a single fixedtime artificial insemination.
OvuGel Indications
For the synchronization of the time of insemination in sows following weaning to facilitate a single fixed-time insemination.
Dosage and Administration
OvuGel should be administered intravaginally to sows approximately 96 hours after weaning. The product should be warmed at room temperature for a minimum of 10 minutes prior to use. Insert the delivery tube into the vagina so that the tip rests 1-2 cm (1/2 inch) posterior to the cervix. Use a separate sheath over the delivery tube for each sow treated. Each sow should receive a single 2 mL dose of OvuGel. Sows should be inseminated 22 ± 2 hours following administration of OvuGel using standard artificial insemination techniques. Sows should be exposed to a boar during time of insemination. (See end of package insert for more detailed directions for OvuGel administration).Use of a bottle mount multi-dose applicator is recommended to ensure accurate dosing.
The use of adequate sperm numbers per insemination and proper semen storage, as recommended by the semen or artificial inseminator catheter suppliers, is recommended.
Illustrated OvuGel Directions for Use
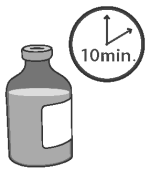
1 Allow the vial of OvuGel to warm to room temperature for a minimum of 10 minutes.
2 Locate the applicator, infusion tube, and protective sheaths to be used. Use of a multi-dose applicator set to deliver 2 mL is recommended.
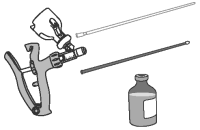
3 Attach infusion tube to applicator.
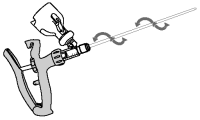
4 Remove foil tab from the vial top. Invert applicator over upright vial of OvuGel and push vial onto the applicator.
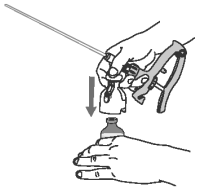
5 Compress the applicator handle fully and then release, allowing applicator chamber to fill with OvuGel.
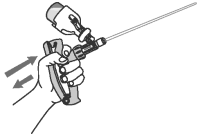
6 Tilt applicator so that infusion tube is pointed up.

7 Compress and release the applicator handle causing the OvuGel within the chamber to enter the infusion tube and another dose from the vial to refill the chamber.
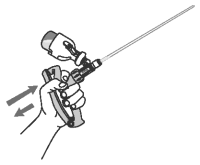
8 Slowly compress and release the applicator handle to displace any air in the infusion tube with OvuGel. There should be a full dose in the infusion tube and in the applicator chamber.

9 Slide the protective sheath over the infusion tube.
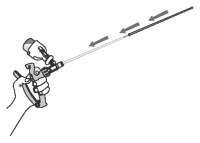
10 Gently and slowly insert the covered infusion tube into the vagina at a slight upper angle (to avoid entry into the urethra) until you encounter mild resistance (the cervix) and then withdraw the infusion tube approximately 1-2 cm (1/2 inch).
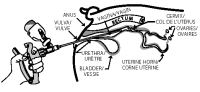
11 Compress and release the applicator handle to discharge the OvuGel dose and to allow another full dose of OvuGel to enter chamber.
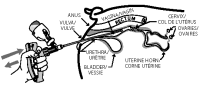
12 Remove the infusion tube and the protective sheath from the vagina. Dispose of the used protective sheath and replace it with a new sheath.
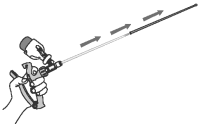
Repeat steps 9 through 12 for each sow receiving OvuGel.
OvuGel should be administered within 1 hour of warming. Unused product maybe stored under refrigeration for up to 28 days from the date the stopper is first punctured. The vial stopper maybe punctured a maximum of three times during that 28 day period.
CAUTIONS: OvuGel should not be used in sows with obvious reproductive tract abnormalities. Not approved for use in gilts as safety and effectiveness have not been evaluated in these animals.
Use may cause minor vaginal inflammation in sows.
Warnings
No withdrawal period is required when used according to label directions. Avoid direct contact with skin or eyes. Wash hands after handling. Keep Out of Reach of Children.EFFICACY STUDIES: A multi-location pivotal study (Study No PTK 9-06) was conducted to demonstrate effectiveness and reproductive safety of OvuGel (200 µg triptorelin (as acetate) per 2 mL dose) to facilitate a single fixed-time insemination approximately 22 hours following treatment in sows following weaning.
Primary effectiveness outcome variables in this study were conception and farrowing rates in sows treated with OvuGel as compared to sows treated with a vehicle gel. Additional variables associated with treatment effectiveness (secondary effectiveness outcome) included pregnancy rate and piglet index, which is the manifestation of both farrowing rate and litter size. Each outcome was assessed using a two sided test at alpha=0.05.
Outcomes associated with reproductive safety included pregnancy failures, gestation length, stillborn pigs per litter, mummified pigs per litter, live litter birth weight, pre-weaning pig mortality per litter, litter weaning weight, post-weaning interval to estrus through day seven after weaning, total pigs per litter, live pigs per litter, and pigs weaned per litter between sows given triptorelin gel versus sows given formulation vehicle gel. These outcomes were assessed using two sided tests at alpha=0.10.
A total of 1,886 healthy postpartum sows (parities 1 to 7) from five breeding herds were randomly assigned to receive either intravaginal vehicle gel (0 µg triptorelin) or OvuGel.
Sows at each site were blocked for parity and lactation length. Breeds included in this study were Newsham hybrid, PIC Line C-22, PIC Line C-29, PIC maternal line and Large White x Landrace. Sows were inseminated approximately 22 ± 2 hours after treatment. A boar was present in front of sows at time of insemination.
In addition to sows randomized to OvuGel and vehicle gel, outcomes from approximately 200 contemporary non-treated sows from each site were collected. This allowed for comparisons to be made to sows inseminated daily for multiple days based on estrous behaviour, as per industry practice. No statistical comparisons were made between treated and contemporary sows, however differences between groups were evaluated using predetermined acceptable differences.
The analysis of effectiveness outcomes, farrowing and conception rates was conducted on data derived from 485 vehicle treated sows and 490 OvuGel treated sows.
The statistical analysis of the effectiveness outcomes for all sows (parities 1 to 7) demonstrated that conception rates were significantly higher (P=0.0195) in OvuGel treated sows (85.63%) as compared to vehicle treated sows (80.04%). Similarly, farrowing rates were significantly higher (P=0.0167 for OvuGel treated sows as compared to vehicle treated sows (84.16% vs 77.11%, respectively).
The piglet Index favoured OvuGel over vehicle gel treated sows.
Reproductive and piglet safety variables: There were no significant treatment effects (P>0.10) between the OvuGel and vehicle treated groups for the following measured variables: pregnancy failure rate, gestation length, number of stillborn piglets per litter, number of mummified piglets per litter, actual and adjusted live litter birth weight, number of live piglets per litter, pre-weaning piglet morality per litter, litter weaning weight, days from weaning to estrus, total piglets per litter, and piglets weaned per litter. No adverse reactions were reported in this study.
When OvuGel treated sows were compared to contemporary sows, the number of inseminations (doses of semen required) was less (1.0 versus 2.1) and live pigs produced per semen dose was greater (9.0 versus 4.8)
Based on the clinical effectiveness demonstrated in the pivotal effectiveness and safety for in-field use data, OvuGel can be safely and effectively used for the synchronization of the time of insemination in sows following weaning to facilitate a single fixed-time insemination.
STORAGE, HANDLING, AND DISPOSAL: Store at refrigerator between 2°C and 8°C.
OvuGel should be administered within 1 hour of warming; unused portions may be refrigerated immediately after use. The vial stopper may be punctured a maximum of three times. Discard 28 days after the stopper is first punctured.
How Supplied
OvuGel is available in 50 mL vials.OvuGel® is a trademark of United-AH, LLC and is used by Vetoquinol under license.
Revised on May 29, 2019.
Vetoquinol N.-A. Inc., 2000, ch. Georges, Lavaltrie, QC, Canada J5T 3S5
103913
|
Net |
Code |
|
|
50 mL |
457548 |
103914, 103912 |
CPN: 1234462.0
Commercial Division
2000, CHEMIN GEORGES, LAVALTRIE, QC, J5T 3S5
| Telephone: | 450-586-2252 | |
| Order Desk: | 800-363-1700 | |
| Fax: | 450-586-4649 | |
| Website: | www.vetoquinol.ca | |
| Email: | info@vetoquinol.ca |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-08-27
