Betamethasone: Package Insert / Prescribing Info
Package insert / product label
Generic name: betamethasone dipropionate
Dosage form: lotion
Drug class: Topical steroids
Medically reviewed by Drugs.com. Last updated on Oct 11, 2024.
On This Page
Betamethasone Description
Betamethasone Dipropionate Lotion, USP 0.05% w/w contains betamethasone dipropionate USP, a synthetic adrenocorticosteroid, for dermatologic use. Betamethasone, an analog of prednisolone, has a high degree of glucocorticoid activity and a slight degree of mineralocorticoid activity. Betamethasone dipropionate is the 17, 21-dipropionate ester of betamethasone.
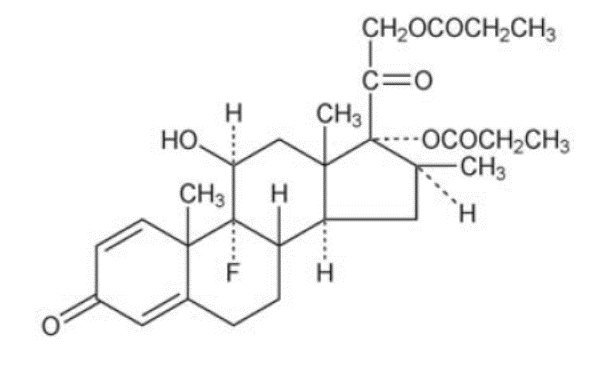
Chemically, betamethasone dipropionate is 9-fluoro-11β,17,21-trihydroxy-16β-methylpregna-1,4-diene-3,20-dione 17,21-dipropionate. The structural formula is:
Molecular Formula: C28H37FO7 Molecular Weight: 504.60
Betamethasone dipropionate is a white to cream white odorless crystalline powder, insoluble in water.
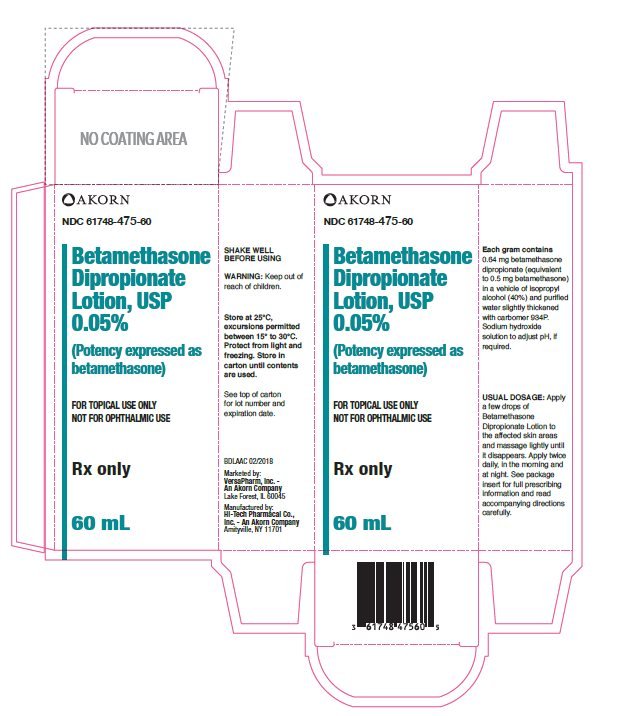
Each gram of Betamethasone Dipropionate Lotion, USP 0.05% w/w contains: 0.643 mg betamethasone dipropionate (equivalent to 0.5 mg betamethasone) in a vehicle of isopropyl alcohol, USP (40.0%) and purified water, USP; slightly thickened with carbomer 934P. Sodium hydroxide solution to adjust pH, if required.
Betamethasone - Clinical Pharmacology
The corticosteroids are a class of compounds comprising steroid hormones, secreted by the adrenal cortex and their synthetic analogs. In pharmacologic doses corticosteroids are used primarily for their anti-inflammatory and/or immunosuppressive effects.
Topical corticosteroids, such as betamethasone dipropionate, are effective in the treatment of corticosteroid-responsive dermatoses primarily because of their anti-inflammatory, antipruritic, and vasoconstrictive actions. However, while the physiologic, pharmacologic, and clinical effects of the corticosteroids are well known, the exact mechanisms of their actions in each disease are uncertain. Betamethasone dipropionate, a corticosteroid, has been shown to have topical (dermatologic) and systemic pharmacologic and metabolic effects characteristic of this class of drugs.
Pharmacokinetics
The extent of percutaneous absorption of topical corticosteroids is determined by many factors including the vehicle, the integrity of the epidermal barrier, and the use of occlusive dressings. (See DOSAGE AND ADMINISTRATION).
Topical corticosteroids can be absorbed from normal intact skin. Inflammation and/or other disease processes in the skin increase percutaneous absorption. Occlusive dressings substantially increase the percutaneous absorption of topical corticosteroids. (See DOSAGE AND ADMINISTRATION.)
Once absorbed through the skin, topical corticosteroids are handled through pharmacokinetic pathways similar to systemically administered corticosteroids. Corticosteroids are bound to plasma proteins in varying degrees. Corticosteroids are metabolized primarily in the liver and are then excreted by the kidneys. Some of the topical corticosteroids and their metabolites are also excreted into the bile.
Twenty-five pediatric patients ages 6 to 12 years, with atopic dermatitis, were enrolled in an open-label, hypothalamic-pituitary-adrenal (HPA) axis safety study. Betamethasone Dipropionate Lotion, USP 0.05% w/w was applied twice daily for 2 to 3 weeks over a mean body surface area of 45% (range 35% to 72%). In 11 of 15 (73%) evaluable patients, adrenal suppression was indicated by either a ≤ 5 mcg/dL pre-stimulation cortisol, or a cosyntropin post-stimulation cortisol ≤ 18 mcg/dL and an increase of < 7 mcg/dL from the baseline cortisol. Studies performed with Betamethasone Dipropionate Lotion, USP 0.05% w/w indicate that it is in the medium range of potency as compared with other topical corticosteroids.
Indications and Usage for Betamethasone
Betamethasone Dipropionate Lotion, USP 0.05% w/w is a medium-potency corticosteroids indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses in patients 13 years and older.
Contraindications
Betamethasone Dipropionate Lotion, USP 0.05% w/w is contraindicated in those patients who are hypersensitive to betamethasone dipropionate, to other corticosteroids, or to any of the components in these preparation.
Precautions
General
Systemic absorption of topical corticosteroids has produced reversible hypothalamic-pituitary-adrenal (HPA) axis suppression, manifestations of Cushing's syndrome, hyperglycemia, and glucosuria in some patients.
Conditions which augment systemic absorption include the application of the more potent steroids, use over large surface areas, prolonged use, and the addition of occlusive dressings. Use of more than one cotrticosteroid-containing product at the same time may increase total systemic glucocorticoid exposure. (See DOSAGE AND ADMINISTRATION)
Therefore, patients receiving a large dose of a potent topical steroid applied to a large surface area should be evaluated periodically for evidence of HPA axis suppression by using the urinary-free cortisol and ACTH stimulation tests. If HPA axis suppression is noted, an attempt should be made to withdraw the drug, to reduce the frequency of application, or to substitute a less potent steroid.
Recovery of HPA axis function is generally prompt and complete upon discontinuation of the drug. In an open-label pediatric study of 15 evaluable patients, of the 11 subjects who showed evidence of suppression, 6 subjects were tested 2 weeks after discontinuation of Betamethasone Dipropionate Lotion, USP 0.05% w/w, and 4 of the 6 (67%) had complete recovery of HPA axis function. Infrequently, signs and symptoms of steroid withdrawal may occur, requiring supplemental systemic corticosteroids.
Pediatric patients may absorb proportionally larger amounts of topical corticosteroids and thus be more susceptible to systemic toxicity. (See PRECAUTIONS - Pediatric Use.)
If irritation develops, topical corticosteroids should be discontinued and appropriate therapy instituted.
In the presence of dermatological infections, the use of an appropriate antifungal or antibacterial agent should be instituted. If a favorable response does not occur promptly, the corticosteroid should be discontinued until the infection has been adequately controlled.
Information for Patients
This information is intended to aid in the safe and effective use of this medication. It is not a disclosure of all possible adverse or intended effects.
Patients using topical corticosteroids should receive the following information and instructions:
1. This medication is to be used as directed by the physician. It is for external use only. Avoid contact with the eyes.
2. Patients should be advised not to use this medication for any disorder other than that for which it was prescribed.
3. The treated skin area should not be bandaged or otherwise covered or wrapped as to be occlusive. (See DOSAGE AND ADMINISTRATION.)
4. Patients should report any signs of local adverse reactions.
5. Other corticosteroid-containing products should not be used with Betamethasone Dipropionate Lotion, USP 0.05% w/w without first talking to your physician.
Laboratory Tests
The following tests may be helpful in evaluating the HPA axis suppression:
Urinary free cortisol test
ACTH stimulation test
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies have not been performed to evaluate the carcinogenic potential of betamethasone dipropionate.
Betamethasone was negative in the bacterial mutagenicity assay (Salmonella typhimurium and Escherichia coli), and in the mammalian cell mutagenicity assay (CHO/HGPRT). It was positive in the in-vitro human lymphocyte chromosome aberration assay, and equivocal in the in-vivo mouse bone marrow micronucleus assay. This pattern of response is similar to that of dexamethasone and hydrocortisone.
Reproductive studies with betamethasone dipropionate carried out in rabbits at doses of 1.0 mg/kg by the intramuscular route and in mice up to 33 mg/kg by the intramuscular route indicated no impairment of fertility except for dose-related increases in fetal resorption rates in both species. These doses are approximately 0.5 and 4 fold the estimated maximum human dose based on a mg/m2 comparison, respectively.
Pregnancy: Teratogenic effects: Pregnancy Category C
Corticosteroids are generally teratogenic in laboratory animals when administered systemically at relatively low dosage levels.
Betamethasone dipropionate has been shown to be teratogenic in rabbits when given by the intramuscular route at doses of 0.05 mg/kg. This dose is approximately 0.03 fold the estimated maximum human dose based on a mg/m2 comparison. The abnormalities observed included umbilical hernias, cephalocele and cleft palates.
Some corticosteroids have been shown to be teratogenic after dermal application in laboratory animals. There are no adequate and well-controlled studies in pregnant women on teratogenic effects from topically applied corticosteroids. Therefore, topical corticosteroids should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Drugs of this class should not be used extensively on pregnant patients, in large amounts, or for prolonged periods of time.
Nursing Mothers
It is not known whether topical administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in breast milk. Systemically administered corticosteroids are secreted into breast milk in quantities not likely to have a deleterious effect on the infant. Nevertheless, caution should be exercised when topical corticosteroids are administered to a nursing woman.
Pediatric Use
Use of Betamethasone Dipropionate Lotion, USP 0.05% w/w in pediatric patients 12 years of age and younger is not recommended. (See CLINICAL PHARMACOLOGY and ADVERSE REACTIONS Sections.) In an open-label study, 11 of 15 (73%) evaluable pediatric patients (aged 6 years-12 years old) using Betamethasone Dipropionate Lotion, USP 0.05% w/w for treatment of atopic dermatitis for 2-3 weeks demonstrated adrenal suppression. (See CLINICAL PHARMACOLOGY - Pharmacokinetics.)
Pediatric patients may demonstrate greater susceptibility to topical corticosteroid-induced HPA axis suppression and Cushing's syndrome than mature patients because of a larger skin surface area to body weight ratio.
Hypothalamic-pituitary-adrenal (HPA) axis suppression, Cushing's syndrome, and intracranial hypertension have been reported in pediatric patients receiving topical corticosteroids. Manifestations of adrenal suppression in pediatric patients include linear growth retardation, delayed weight gain, low plasma cortisol levels, and absence of response to ACTH stimulation. Manifestations of intracranial hypertension include bulging fontanelles, headaches, and bilateral papilledema.
Administration of topical corticosteroids to pediatric patients should be limited to the least amount compatible with an effective therapeutic regimen. Chronic corticosteroid therapy may interfere with the growth and development of pediatric patients.
Adverse Reactions/Side Effects
The following local adverse reactions are reported infrequently when Betamethasone Dipropionate Lotion, USP 0.05% w/w is used as recommended in the DOSAGE AND ADMINISTRATIONsection. These reactions are listed in an approximate decreasing order of occurrence: burning, itching, irritation, dryness, folliculitis, hypertrichosis, acneiform eruptions, hypopigmentation, perioral dermatitis, allergic contact dermatitis, maceration of the skin, secondary infections, skin atrophy, striae and miliaria.
Adverse reactions reported to be possibly or probably related to treatment with Betamethasone Dipropionate Lotion, USP 0.05% w/w during a pediatric study include: paresthesia (burning), erythema, erythematous rash, and dry skin. These adverse reactions each occurred in a different patient; 4% of the 25 patient population, respectively. An adverse reaction reported to be possibly or probably related to treatment in 2 different patients, 8%, of the 25 patients is puritis.
Systemic absorption of topical corticosteroids has produced reversible hypothalamic-pituitary-adrenal (HPA) axis suppression, manifestations of Cushing's syndrome, hyperglycemia and glucosuria in some patients.
Related/similar drugs
Skyrizi
Skyrizi (risankizumab) is used to treat plaque psoriasis, psoriatic arthritis, ulcerative colitis ...
Dupixent
Dupixent is used to treat eczema, eosinophilic or oral-corticosteroid-dependent asthma, chronic ...
Otezla
Otezla (apremilast) is used to treat plaque psoriasis, psoriatic arthritis, and oral ulcers ...
Stelara
Stelara (ustekinumab) is used to treat Crohn's disease, ulcerative colitis, plaque psoriasis, and ...
Cosentyx
Cosentyx (secukinumab) is used to treat plaque psoriasis, psoriatic arthritis, ankylosing ...
Humira
Humira is a tumor necrosis factor blocker used to treat many inflammatory conditions in adults ...
Ilumya
Ilumya (tildrakizumab) is used to treat moderate-to-severe plaque psoriasis to reduce plaques ...
Taltz
Taltz (ixekizumab) is used to treat plaque psoriasis, psoriatic arthritis, and ankylosing ...
Temovate
Temovate is used for atopic dermatitis, dermatitis, dermatological disorders, eczema, lichen ...
Overdosage
Topically applied corticosteroids can be absorbed in sufficient amounts to produce systemic effects. (See PRECAUTIONS.)
Betamethasone Dosage and Administration
Apply a few drops of Betamethasone Dipropionate Lotion to the affected areas and massage lightly until it disappears. Apply twice daily, in the morning and at night. For the most effective and economical use, apply nozzle very close to affected area and gently squeeze bottle.
Betamethasone Dipropionate Lotion, USP 0.05% w/w is not to be used with occlusive dressings.
How is Betamethasone supplied
Betamethasone Dipropionate Lotion, USP 0.05% w/w is supplied as follows:
60 mL bottles (NDC 61748-475-60)
Store at 25°C, excursions permitted between 15° and 30°C. Protect from light and freezing. Store in carton until contents are used.
VersaPharm Inc. – An Akorn Company
Lake Forest, IL 60045
Manufactured by:
Hi-Tech Pharmacal Co. Inc. – An Akorn Company
Amityville, NY 11701
BDL00N
Rev. 02/2018
| BETAMETHASONE DIPROPIONATE
betamethasone dipropionate lotion |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Versapharm Incorporated (117696953) |
| Registrant - Akorn Operating Company LLC (117693100) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Hi-Tech Pharmacal Co., Inc. | 117696873 | MANUFACTURE(61748-475) , PACK(61748-475) , ANALYSIS(61748-475) | |
More about betamethasone topical
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (56)
- Side effects
- Dosage information
- Patient tips
- During pregnancy
- Drug class: topical steroids
- Breastfeeding
- En español
Patient resources
- Betamethasone Topical drug information
- Betamethasone dipropionate (Advanced Reading)
- Betamethasone valerate (Advanced Reading)
Professional resources
- Betamethasone, Betamethasone Benzoate, Betamethasone Dipropionate, Betamethasone Valerate (Topical) monograph
- Betamethasone Cream (FDA)
- Betamethasone Cream Augmented (FDA)
- Betamethasone Dipropionate (FDA)
- Betamethasone Gel (FDA)
Other brands
Diprolene, Sernivo, Diprolene AF