Twinject Auto-Injector: Package Insert / Prescribing Info
Package insert / product label
Generic name: epinephrine
Dosage form: injection
Drug classes: Adrenergic bronchodilators, Catecholamines, Vasopressors
Medically reviewed by Drugs.com. Last updated on Mar 25, 2025.
The Twinject brand name has been discontinued in the U.S. If generic versions of this product have been approved by the FDA, there may be generic equivalents available.
On This Page
Available as: 0.3 mg 0.15 mg
each dose delivers 0.15 mg or 0.3 mg of epinephrine
PRESCRIBING INFORMATION
Twinject Auto-Injector Description
Twinject auto-injector contains 1.1 mL epinephrine injection, USP 1:1000 (1 mg/mL), from which two doses of either 0.15 mg (0.15 mL) or 0.3 mg (0.3 mL) each are available for use by injection. The first dose is administered by auto-injection after the patient prepares and fires Twinject as directed. A second dose can be manually administered following a partial disassembly of Twinject. The remaining volume is not available for use and should be discarded. See PATIENT DIRECTIONS FOR USE on the accompanying Patient Information Leaflet.
Each dose of epinephrine injection, USP 1:1000 contains either 0.15 mg or 0.3 mg l-epinephrine, sodium chloride, chlorobutanol and sodium bisulfite, all sealed under nitrogen.
Epinephrine is a sympathomimetic catecholamine. Its naturally occurring l-isomer, which is twenty times as active as the d-isomer, is obtained in pure form by separation from the synthetically produced racemate.
Chemically, epinephrine is 1-(3,4-dihydroxyphenyl)-2-(methylamino)ethanol with the following structure:
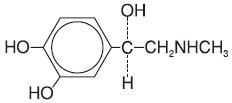
Epinephrine deteriorates rapidly on exposure to air or light, turning pink from oxidation to adrenochrome and brown from the formation of melanin. Epinephrine solutions that show evidence of discoloration should be discarded.
Twinject contains no latex.
Twinject Auto-Injector - Clinical Pharmacology
Epinephrine is the drug of choice for the emergency treatment of severe allergic reactions (Type I) to allergens, such as those present in certain insect venoms, foods, or drugs. It can also be used in the treatment of anaphylaxis of unknown cause (idiopathic anaphylaxis) or exercise-induced anaphylaxis. Epinephrine, when given intramuscularly or subcutaneously, has a rapid onset and short duration of action. Epinephrine acts on both alpha and beta adrenergic receptors. Through its action on alpha adrenergic receptors, epinephrine lessens the vasodilation and increased vascular permeability that occurs during an anaphylactic reaction and can lead to loss of intravascular fluid volume and hypotension. Through its action on beta adrenergic receptors, epinephrine causes bronchial smooth muscle relaxation that helps alleviate bronchospasm, wheezing, and dyspnea that may occur during anaphylaxis. Epinephrine also helps alleviate pruritus, urticaria, and angioedema, and may be effective in relieving gastrointestinal and genitourinary symptoms of anaphylaxis because of its relaxer effects on the smooth muscle of the stomach, intestine, uterus and urinary bladder.
Indications and Usage for Twinject Auto-Injector
Twinject (epinephrine injection, USP 1:1000) is indicated in the emergency treatment of severe allergic reactions (Type I) including anaphylaxis to stinging insects (e.g. order Hymenoptera, which includes bees, wasps, hornets, yellow jackets and fire ants), and biting insects (e.g. triatoma, mosquitos), allergen immunotherapy, foods, drugs, diagnostic testing substances (e.g. radiocontrast media), and other allergens, as well as anaphylaxis to unknown substances (idiopathic anaphylaxis) or exercise-induced anaphylaxis. Twinject is intended for immediate administration in patients with a history of anaphylactic reactions. Selection of the appropriate dosage strength is determined according to patient body weight (See DOSAGE AND ADMINISTRATION section).
Such reactions may occur within minutes after exposure and consist of flushing, apprehension, syncope, tachycardia, thready or unobtainable pulse associated with a fall in blood pressure, convulsions, vomiting, diarrhea and abdominal cramps, involuntary voiding, wheezing, dyspnea due to laryngeal spasm, pruritus, rashes, urticaria, or angioedema. Twinject is designed as emergency supportive therapy only and is not a replacement or substitute for immediate medical care.
Contraindications
There are no absolute contraindications to the use of epinephrine in a life-threatening allergic reaction.
Warnings
Twinject should only be injected into the anterolateral aspect of the thigh. Accidental injection into the hands or feet may result in loss of blood flow to the affected area and should be avoided. DO NOT INJECT INTO BUTTOCK. If there is an accidental injection into these areas, advise the patient to inform the healthcare provider of the accidental injection when he/she goes to the nearest emergency room for further treatment of anaphylaxis.
Avoid possible inadvertent intravascular administration. Large doses or accidental intravenous injection of epinephrine may result in cerebral hemorrhage due to a sharp rise in blood pressure. DO NOT INJECT INTRAVENOUSLY. Rapidly acting vasodilators can counteract the marked pressor effects of epinephrine if there is such inadvertent administration.
Epinephrine is the preferred treatment for serious allergic reactions or other emergency situations even though this product contains sodium bisulfite, a sulfite that may, in other products, cause allergic-type reactions including anaphylactic symptoms or life-threatening or less severe asthmatic episodes in certain susceptible persons. The alternatives to using epinephrine in a life-threatening situation may not be satisfactory. The presence of a sulfite in this product should not deter administration of the drug for treatment of serious allergic or other emergency situations, even if the patient is sulfite-sensitive.
Epinephrine should be administered with caution to patients with cardiac arrhythmias, coronary artery or organic heart disease, or hypertension. In patients with coronary insufficiency or ischemic heart disease, epinephrine may precipitate or aggravate angina pectoris as well as produce ventricular arrhythmias. It should be recognized that the presence of these conditions is not a contraindication to epinephrine administration in an acute, life-threatening situation.
Epinephrine is light sensitive and should be stored in the carrying-case provided. Store at room temperature (20°-25°C/68°-77°F) with excursions permitted to 15°-30°C (59°-86°F). Do not refrigerate; protect from freezing. Patients should periodically check the solution in Twinject for any discoloration and/or precipitates. If the solution is discolored or contains a precipitate, the patient should replace their Twinject.
Precautions
(1) General
Twinject is not intended as a substitute for immediate medical care. In conjunction with the administration of epinephrine, the patient should seek appropriate medical care. More than two sequential doses of epinephrine should only be administered under direct medical supervision.
Twinject is not suitable for patients, or caregivers, with such disabilities as severe debilitating arthritis of the hands, because the use of this product requires some manual dexterity to administer. IN ALL CASES, THE PHYSICIAN SHOULD INSTRUCT THE PATIENT AND/OR ANY OTHER PERSON WHO MIGHT BE IN A POSITION TO ADMINISTER THE EPINEPHRINE, IN THE PROPER USE OF Twinject.
Epinephrine is essential for the treatment of anaphylaxis. Patients with a history of severe allergic reactions should be instructed about the circumstances under which epinephrine should be used (See INDICATIONS AND USAGE Section). It should be determined that the patient is at risk of future anaphylaxis, since there are some concerns in specific patients with epinephrine administration. (a) Epinephrine should be used with caution in patients with cardiac arrhythmias, coronary artery or organic heart disease, hypertension, or in patients who are on medications that may sensitize the heart to arrhythmias, e.g., digitalis, diuretics, or anti-arrhythmics. In such patients, epinephrine may precipitate or aggravate angina pectoris as well as produce ventricular arrhythmias. (b) The effects of epinephrine may be potentiated by tricyclic antidepressants and monoamine oxidase inhibitors. (c) Some patients may be at greater risk of developing adverse reactions after epinephrine administration. These include patients with hyperthyroidism, cardiovascular disease, hypertension, diabetes, and elderly individuals, and pregnant women. It must be noted that, despite these concerns, epinephrine is essential for the treatment of anaphylaxis. Therefore, patients with these conditions, or any other person who might be in a position to administer epinephrine to a patient with these conditions experiencing anaphylaxis, should be instructed about the circumstances under which epinephrine should be used.
(2) Information for Patients
Complete patient information, including dosage, directions for proper administration, and precautions, can be found inside each Twinject package within the Patient Information Leaflet.
Epinephrine may produce symptoms and signs that include an increase in pulse rate, the sensation of a more forceful heartbeat, palpitations, a throbbing headache, pallor, feelings of overstimulation, anxiety, weakness, shakiness, dizziness, or nausea. These signs and symptoms usually subside rapidly, especially with rest, quiet, and recumbency.
Patients with hypertension or hyperthyroidism may develop more severe or persistent effects, and patients with coronary artery disease could experience angina. Patients with diabetes may develop increased blood glucose levels following epinephrine administration. Patients with Parkinson's disease may notice a temporary worsening of symptoms.
(3) Drug Interactions
Patients who receive epinephrine while concomitantly taking cardiac glycosides or diuretics should be observed carefully for the development of cardiac arrhythmias.
The effects of epinephrine may be potentiated by tricyclic antidepressants, monoamine oxidase inhibitors, sodium levothyroxine, and certain antihistamines, notably chlorpheniramine, tripelennamine, and diphenhydramine.
The cardiostimulating and bronchodilating effects of epinephrine are antagonized by beta-adrenergic blocking drugs, such as propranolol. The vasoconstricting and hypertensive efffects are antagonized by alpha-adrenergic blocking drugs, such as phentolamine. Ergot alkaloids and phenothiazines may also reverse the pressor effects of epinephrine.
(4) Carcinogenesis, Mutagenesis, Impairment of Fertility
There are no data from either animal or human studies regarding the carcinogenicity or mutagenicity of epinephrine, and no studies have been conducted to determine its potential for the impairment of fertility. This should not prevent the use of epinephrine under the conditions noted under INDICATIONS AND USAGE section.
(5) Pregnancy
Pregnancy Category C
Epinephrine has been shown to have developmental effects in rabbits at a subcutaneous dose of 1.2 mg/kg (approximately 30 times the maximum recommended daily subcutaneous or intramuscular dose on a mg/m2 basis), in mice at a subcutaneous dose of 1 mg/kg (approximately 7 times the maximum recommended daily subcutaneous or intramuscular dose on a mg/m2 basis), and in hamsters at a subcutaneous dose of 0.5 mg/kg (approximately 5 times the maximum recommended daily subcutaneous or intramuscular dose on a mg/m2 basis). These effects were not seen in mice at a subcutaneous dose of 0.5 mg/kg (approximately 3 times the maximum recommended daily subcutaneous or intramuscular dose on a mg/m2 basis). Although there are no adequate and well-controlled studies in pregnant women, epinephrine crosses the placenta and could lead to fetal anoxia, spontaneous abortion or both. Therefore, epinephrine should be used in pregnancy only if the potential benefit justifies the potential risk to the fetus.
Adverse Reactions/Side Effects
Adverse reactions to epinephrine include transient, moderate anxiety; apprehensiveness; restlessness; tremor; weakness; dizziness; sweating; palpitations; pallor; nausea and vomiting; headache, and/or respiratory difficulties. These symptoms occur in some persons receiving therapeutic doses of epinephrine, but are more likely to occur in patients with hypertension or hyperthyroidism. Large doses of epinephrine can cause acute hypertension. Arrhythmias, including fatal ventricular fibrillation, have been reported, particularly in patients with underlying cardiac disease or those receiving certain drugs [see (3) Drug Interactions]. Rapid rises in blood pressure have produced cerebral hemorrhage, particularly in elderly patients with cardiovascular disease. Angina may occur in patients with coronary artery disease. The potential for epinephrine to produce these types of adverse reactions does not contraindicate its use in an acute, life-threatening allergic reaction.
Related/similar drugs
Overdosage
Epinephrine is rapidly inactivated in the body, and treatment following overdose with epinephrine is primarily supportive. If necessary, pressor effects may be counteracted by rapidly acting vasodilators or alpha-adrenergic blocking drugs. If prolonged hypotension follows such measures, it may be necessary to administer another pressor drug.
Overdosage of epinephrine may produce extremely elevated arterial pressure, which may result in cerebrovascular hemorrhage, particularly in elderly patients.
If an epinephrine overdose induces pulmonary edema that interferes with respiration, treatment consists of a rapidly acting alpha-adrenergic blocking drug and/or respiratory support.
Epinephrine overdosage can also cause transient bradycardia followed by tachycardia, and these may be accompanied by potentially fatal cardiac arrhythmias. Premature ventricular contractions may appear within one minute after injection and may be followed by multifocal ventricular tachycardia (prefibrillation rhythm). Subsidence of the ventricular effects may be followed by atrial tachycardia and occasionally by atrioventricular block. Treatment of arrhythmias consists of administration of a beta-adrenergic blocking drug such as propranolol.
Overdosage sometimes results in extreme pallor and coldness of the skin, metabolic acidosis, and kidney failure. Suitable corrective measures must be taken in such situations.
Twinject Auto-Injector Dosage and Administration
The physician who prescribes Twinject should review this Prescribing Information insert in detail with the patient. This review should include the proper use of Twinject to ensure that subcutaneous or intramuscular injections are given into the anterolateral aspect of the thigh, through clothing if necessary. The accompanying Patient Information Leaflet and Wrap Label should also be reviewed with the patient.
Twinject is capable of delivering two doses of either 0.15 mg or 0.3 mg (0.15 mL or 0.3 mL of 1:1000 dilution of epinephrine) each. The first dose is available for auto-injection by the patient, and the second dose is available for manual injection by the patient following a partial disassembly of Twinject.
Selection of the appropriate Twinject dosage strength is determined according to patient body weight.
| Twinject 0.15 mg | For use by patients who weigh 15 - 30 kilograms (approximately 33 - 66 pounds) |
| Twinject 0.3 mg | For use by patients who weigh 30 kilograms (approximately 66 pounds) or greater |
The usual dose of epinephrine for allergic emergencies in patients who weigh 30 kilograms or greater is 0.3 mg (0.3 mL of 1:1000 dilution of epinephrine).
Since the doses of epinephrine delivered from Twinject are fixed, the physician should consider other forms of injectable epinephrine if doses lower than those available from Twinject are felt to be necessary. The prescribing physician should carefully assess each patient to determine the most appropriate dose of epinephrine, recognizing the life-threatening nature of the reactions for which this drug is being prescribed.
Patients should be instructed to periodically visually inspect the epinephrine solution for particulate matter and discoloration. If the solution contains particulate matter or develops a pinkish color or becomes darker than slightly yellow, the patient should immediately contact their physician for a replacement, since these changes indicate that the effectiveness of the drug product may be decreased.
How is Twinject Auto-Injector supplied
Twinject is a patient (or caregiver) actuated, dual-dose product that contains 1.1 mL of epinephrine injection, USP (1:1000 or 1 mg/mL), of which an initial dose can be delivered by auto-injection, and a second dose is available by manual administration. THE REMAINING VOLUME THAT IS LEFT AFTER THESE TWO FIXED DOSES CANNOT BE FURTHER ADMINISTERED AND SHOULD BE DISCARDED WITH THE DEVICE AS OUTLINED IN THE PATIENT INFORMATION LEAFLET.
Twinject 0.15 mg is available in a single unit carton, NDC 59630-801-01, and in a Two-Pack, NDC 59630-801-02, containing two Twinject 0.15 mg auto-injectors and one Twinject Demonstrator.
Twinject 0.3 mg is available in a single unit carton, NDC 59630-802-01, and in a Two-Pack, NDC 59630-802-02, containing two Twinject 0.3 mg auto-injectors and one Twinject Demonstrator.
Manufactured for and Distributed by: Shionogi Pharma, Inc., Atlanta, GA 30328
©2010 Shionogi Pharma, Inc., Atlanta, GA. All rights reserved. This product may be covered by some or all of the following patents, patent applications and foreign equivalents thereof: U.S. Patent Nos. 5,358,489; 5,540,664; 5,665,071; and 7,297,136 and other pending U.S. Patent applications.
Printed in USA
Revised January, 2010
For inquiries call 1-888-TWIN-JCT
PRINCIPAL DISPLAY PANEL - 0.15 mg Carton Label
Rx Only
Contains One Twinject® 0.15mg Auto-Injector
NDC 59630-801-01
Two doses in each
Twinject® auto-injector
For Subcutaneous or
Intramuscular Use Only
Twinject®
auto-injector
(epinephrine injection, USP 1:1000)
each dose delivers 0.15 mg of epinephrine
Manufactured for and Distributed by:
Shionogi Pharma, Inc.
Atlanta, GA 30328 USA
SHIONOGI PHARMA, INC.
For Allergic Emergencies (Anaphylaxis)
Patient Reminder Program, See Details Inside

PRINCIPAL DISPLAY PANEL - 0.3 mg Carton Label
Rx Only
Contains One Twinject® 0.3mg Auto-Injector
NDC 59630-802-01
Two doses in each
Twinject® auto-injector
For Subcutaneous or
Intramuscular Use Only
Twinject®
auto-injector
(epinephrine injection, USP 1:1000)
each dose delivers 0.3 mg of epinephrine
Manufactured for and Distributed by:
Shionogi Pharma, Inc.
Atlanta, GA 30328 USA
SHIONOGI PHARMA, INC.
For Allergic Emergencies (Anaphylaxis)
Patient Reminder Program, See Details Inside

| TWINJECT
epinephrine injection |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| TWINJECT
epinephrine injection |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Shionogi Pharma, Inc. (802728477) |
Frequently asked questions
- Can you use an expired EpiPen in an emergency?
- Norepinephrine vs epinephrine: what's the difference?
- Can you bring an EpiPen on a plane?
- What's the mechanism of action for epinephrine?
- Does epinephrine cause vasoconstriction?
- How much does Auvi-Q cost compared to EpiPen?
- How does neffy work?
More about Twinject (epinephrine)
- Check interactions
- Compare alternatives
- Latest FDA alerts (3)
- Side effects
- Dosage information
- During pregnancy
- Drug class: adrenergic bronchodilators
- Breastfeeding
Patient resources
Professional resources
Other brands
EpiPen, Adrenalin, neffy, Auvi-Q, ... +4 more
