Hexatrione: Package Insert / Prescribing Info
Package insert / product label
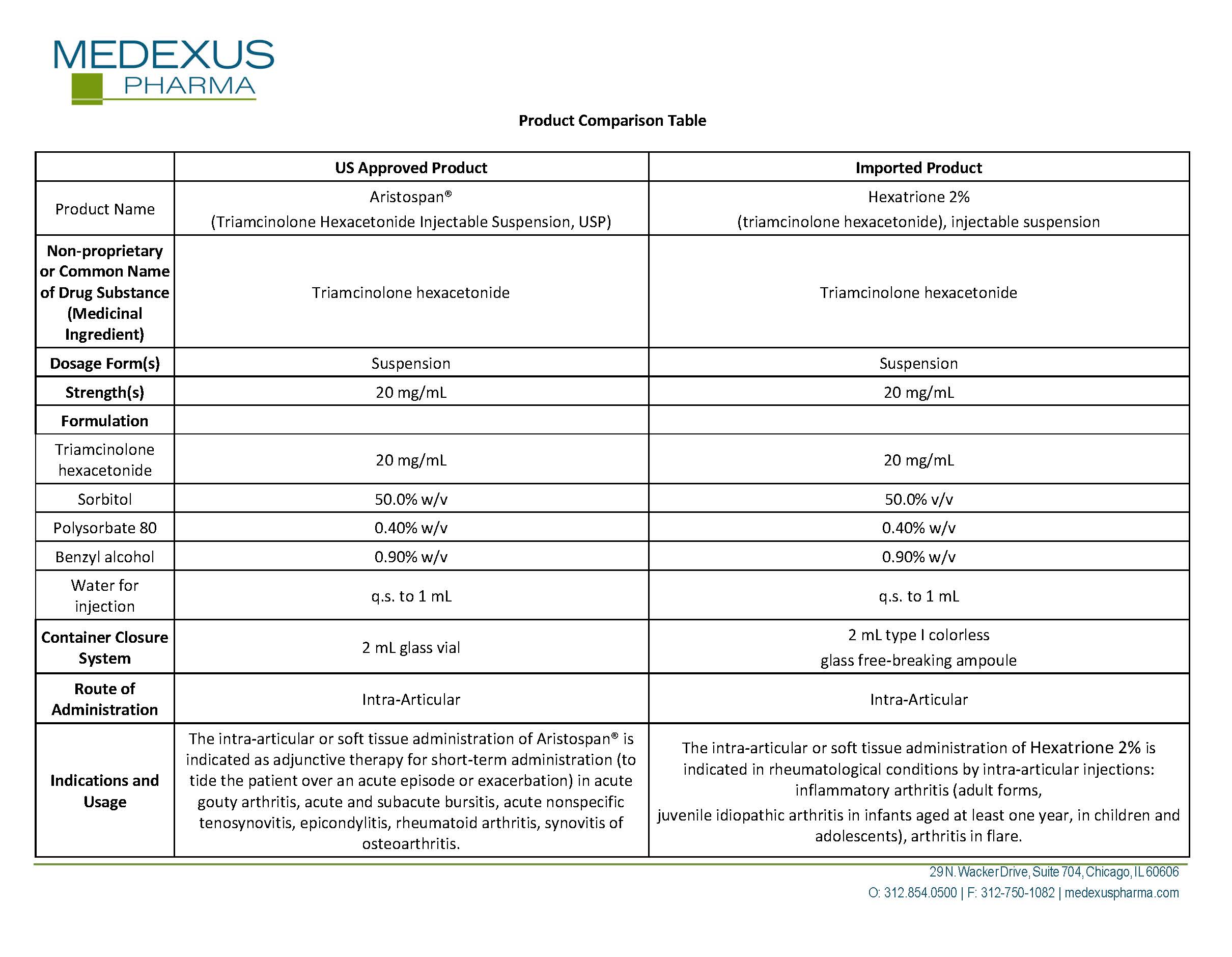
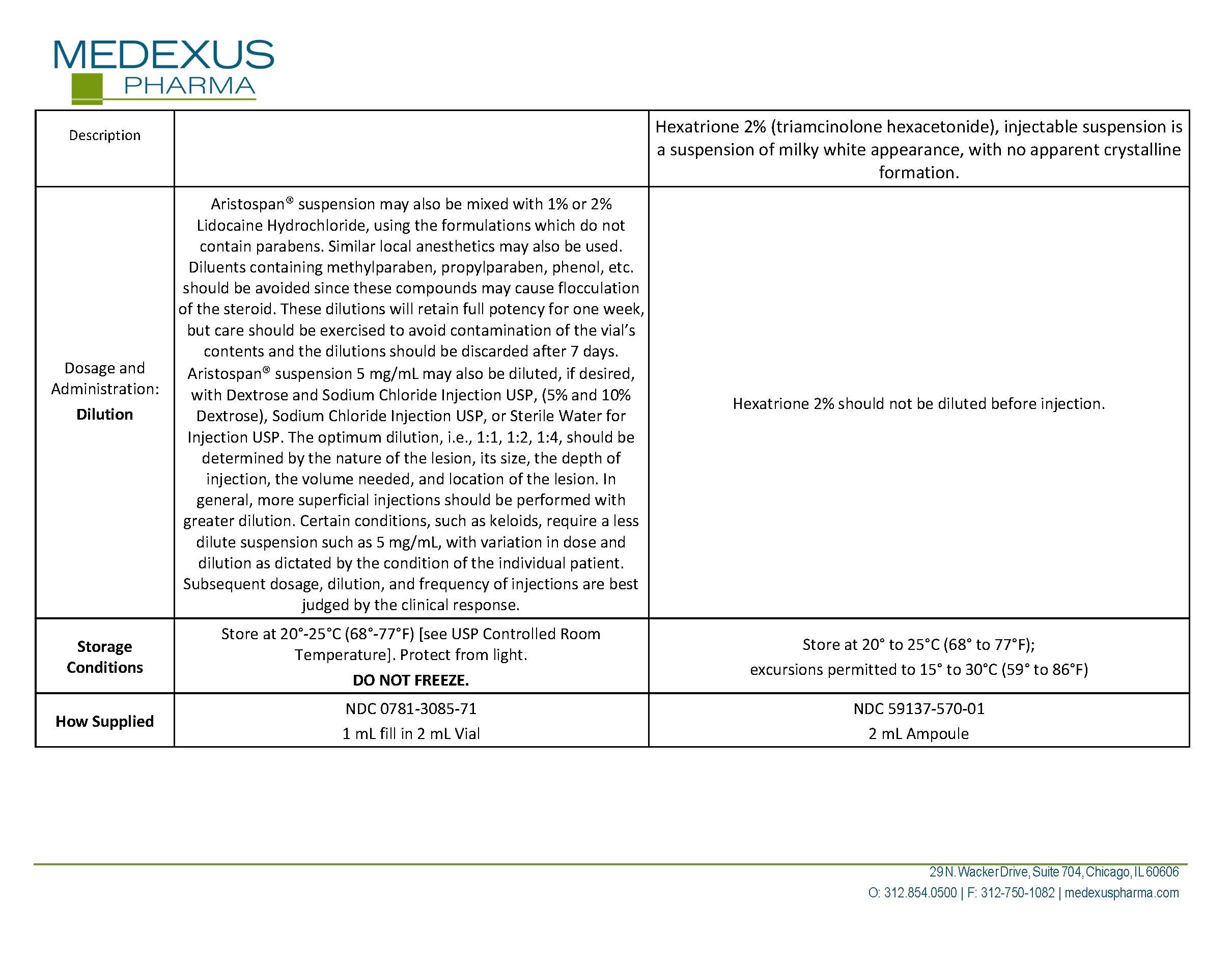
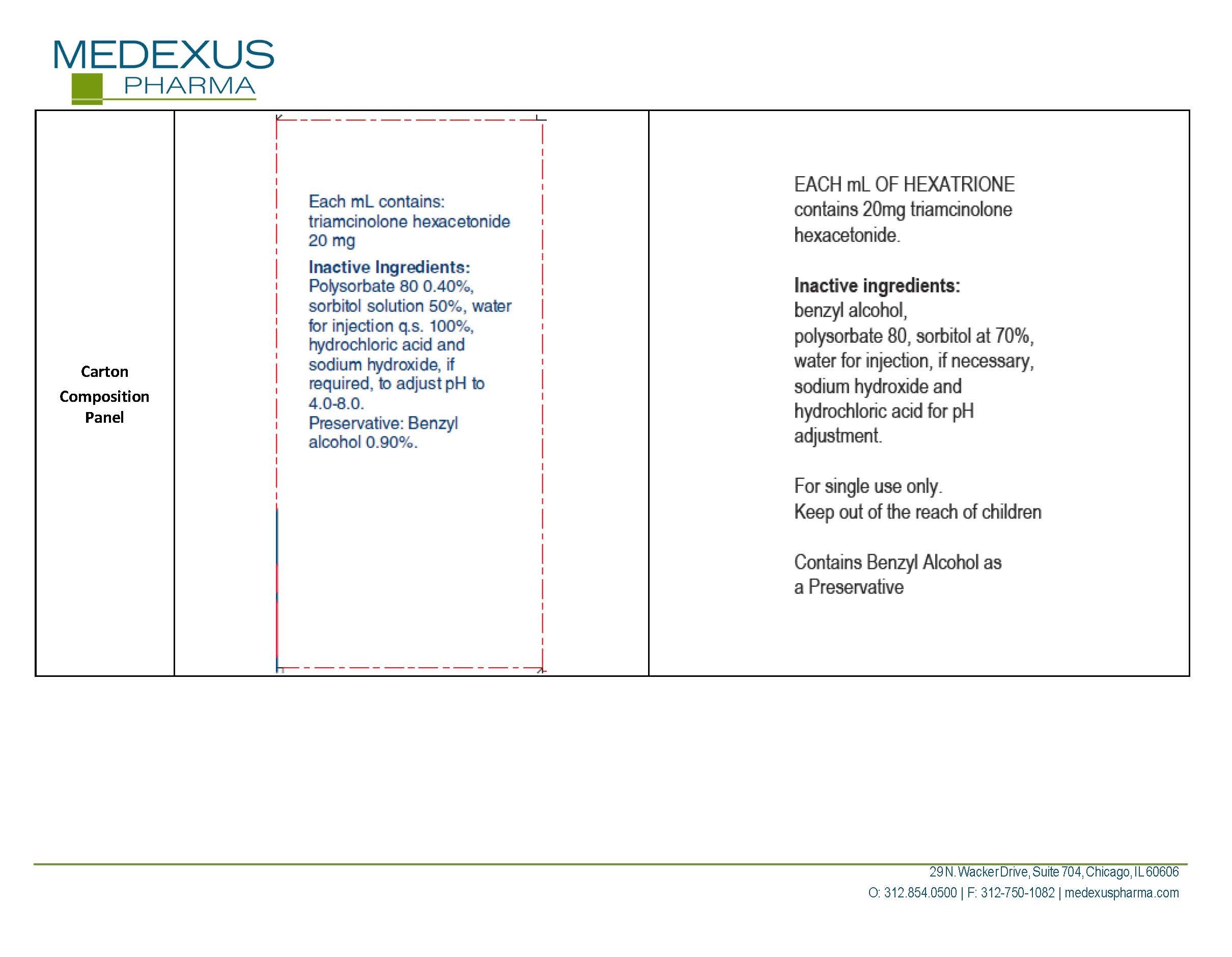
Generic name: triamcinolone hexacetonide
Dosage form: injection, suspension
Drug class: Glucocorticoids
J Code (medical billing code): J3303 (Per 5 mg, injection)
Medically reviewed by Drugs.com. Last updated on Oct 4, 2023.
On This Page
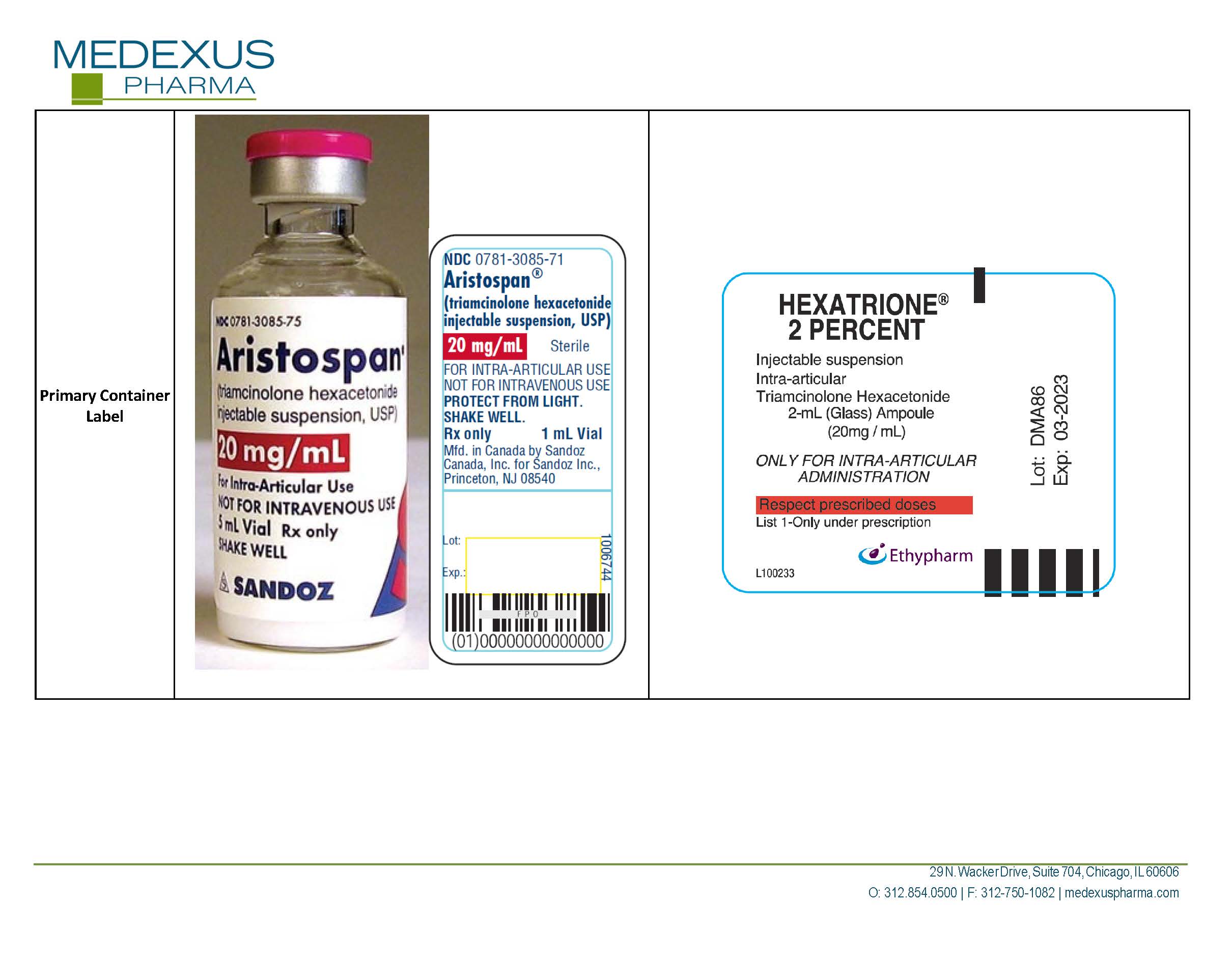
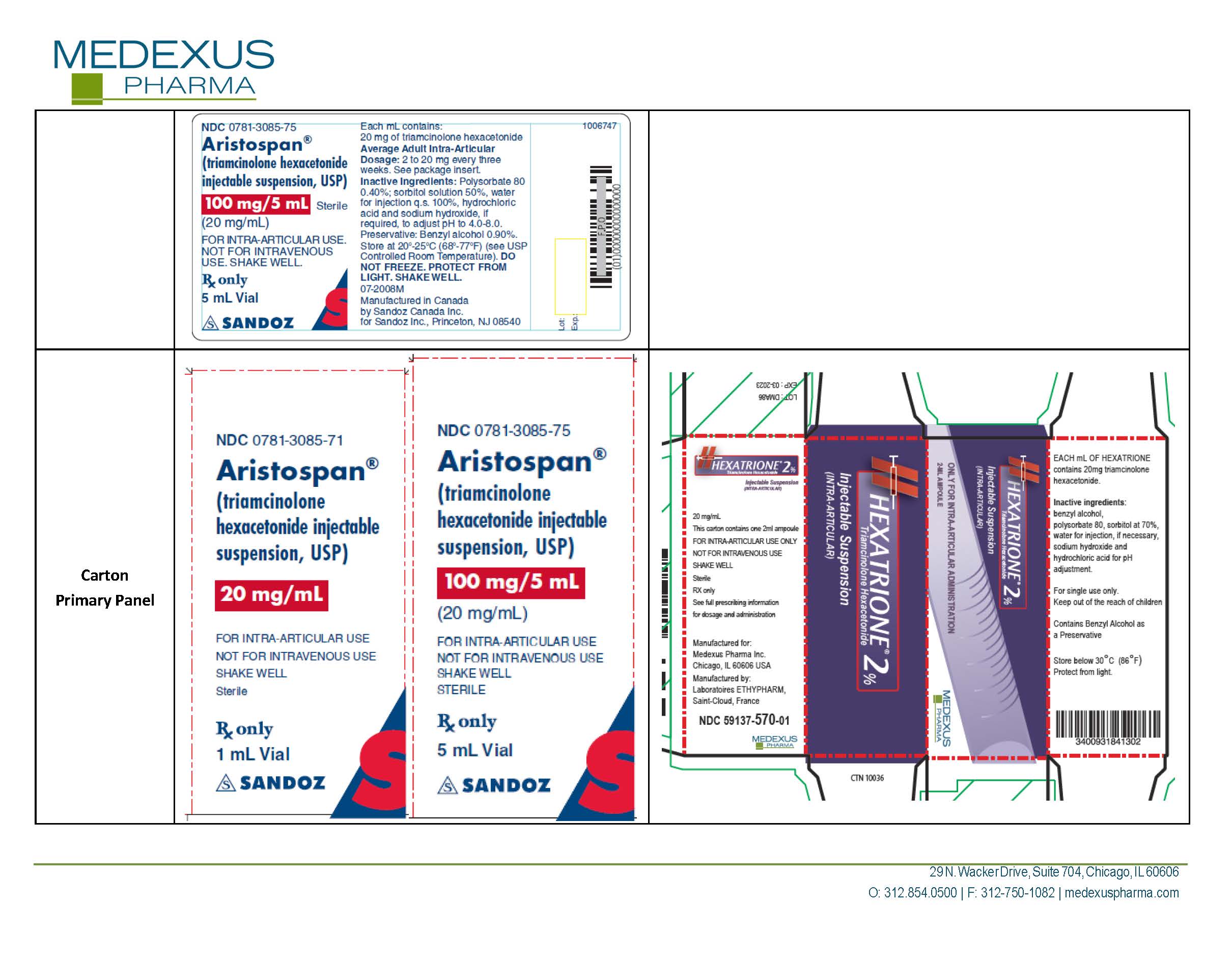
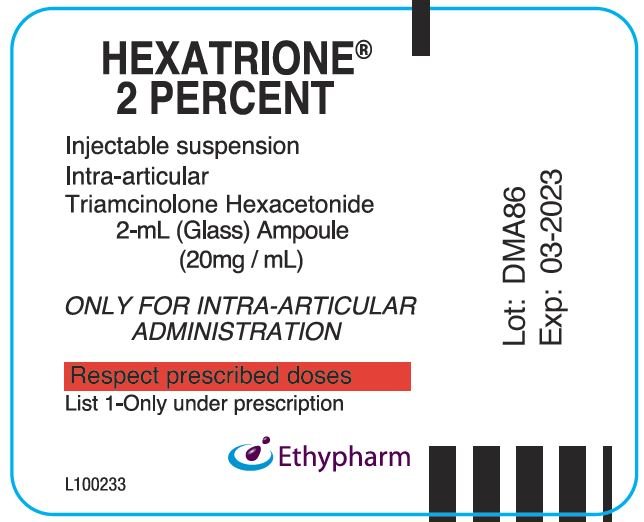
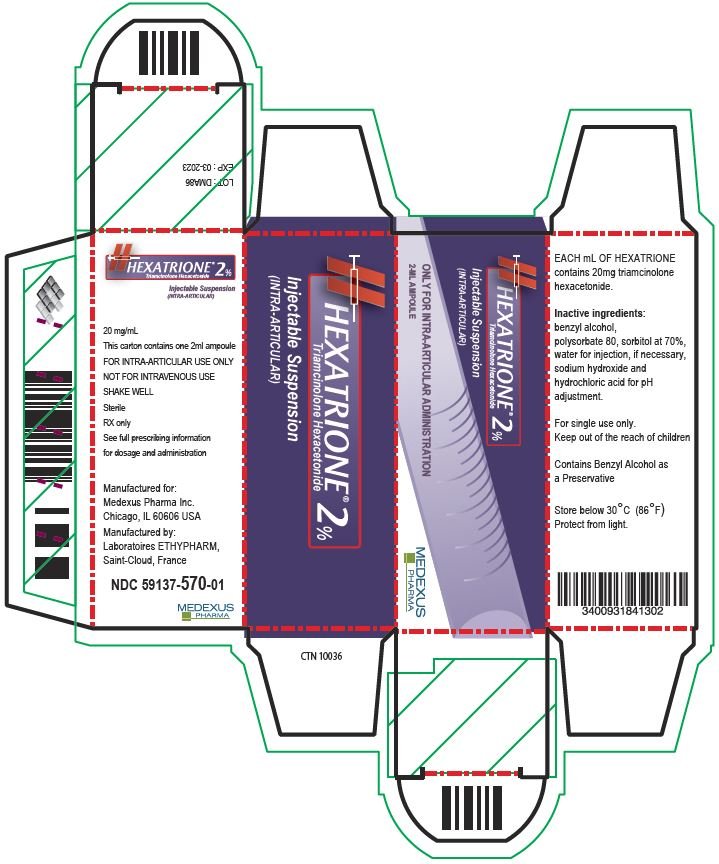
| HEXATRIONE
2%
triamcinolone hexacetonide injection, suspension |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Medexus Pharma, Inc. (078811131) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Valdepharm | 260128560 | manufacture(59137-570) | |
Related/similar drugs
Frequently asked questions
- Where should you not use triamcinolone acetonide cream?
- What causes Plaque Psoriasis?
- Is triamcinolone acetonide an antifungal cream?
- Is triamcinolone good for poison ivy?
- Clobetasol vs. triamcinolone - how do they compare?
- What are steroid injections (cortisone shots)?
- Halobetasol vs triamcinolone: which is better?
- Can triamcinolone acetonide B be used to treat severe diaper rash on a little girl?
More about triamcinolone
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (230)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Support group
- Drug class: glucocorticoids
- Breastfeeding
Patient resources
- Triamcinolone drug information
- Triamcinolone injection
- Triamcinolone (Intra-articular) (Advanced Reading)
Professional resources
Other brands
Kenalog-40, Zilretta, Kenalog-10