Diazoxide: Package Insert / Prescribing Info
Package insert / product label
Dosage form: oral suspension
Drug classes: Agents for hypertensive emergencies, Glucose elevating agents
Medically reviewed by Drugs.com. Last updated on Aug 25, 2025.
On This Page
Diazoxide Description
Diazoxide Oral Suspension, USP is a nondiuretic benzothiadiazine derivative taken orally for the management of symptomatic hypoglycemia. Diazoxide Oral Suspension, USP contains 50 mg of diazoxide in each milliliter and has a chocolate-mint flavor; alcohol content is approximately 7.25%. Other ingredients include sorbitol solution, chocolate mint flavor, propylene glycol, magnesium aluminum silicate, carboxymethylcellulose sodium, sodium benzoate, methylparaben, poloxamer 188, propylparaben, and purified water. Hydrochloric acid or sodium hydroxide may be added to adjust pH.
Diazoxide has the following structural formula:
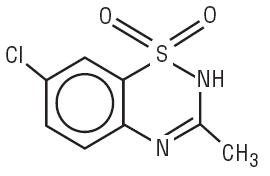
Diazoxide is 7-chloro-3-methyl-2H-1,2,4-benzothiadiazine 1,1-dioxide with the empirical formula C8H7ClN2O2S and the molecular weight 230.7. It is a white powder practically insoluble to sparingly soluble in water.
Diazoxide - Clinical Pharmacology
Diazoxide produces a dose-related increase in blood glucose, due primarily to an inhibition of insulin release from the pancreas, and also to an extrapancreatic effect.
The hyperglycemic effect of diazoxide begins within an hour and generally lasts no more than eight hours in the presence of normal renal function.
Diazoxide decreases the excretion of sodium and water, resulting in fluid retention which may be clinically significant.
The hypotensive effect of diazoxide is usually not marked (see ADVERSE REACTIONS).
Other pharmacologic actions of diazoxide include increased pulse rate; increased serum uric acid levels due to decreased excretion; increased serum levels of free fatty acids' decreased chloride excretion; decreased para-aminohippuric acid; (PAH) clearance with no appreciable effect on glomerular filtration rate.
The concomitant administration of a benzothiazide diuretic may intensify the hyperglycemic and hyperuricemic effects of diazoxide. In the presence of hypokalemia, hyperglycemic effects are also potentiated.
Diazoxide-induced hyperglycemia is reversed by the administration of insulin or tolbutamide. The inhibition of insulin release by diazoxide is antagonized by alpha-adrenergic blocking agents.
Diazoxide is extensively bound (more than 90%) to serum proteins, and is excreted in the kidneys. The plasma half-life following intravenous administration is 28 ± 8.3 hours. Limited data on oral administration revealed a half-life of 24 and 36 hours in two adults. In four children aged four months to six years, the plasma half-life varied from 9.5 to 24 hours on long-term oral administration. The half-life may be prolonged following overdosage, and in patients with impaired renal function.
Indications and Usage for Diazoxide
Diazoxide Oral Suspension is indicated for the management of hypoglycemia due to hyperinsulinism associated with the following conditions:
- Adults: Inoperable islet cell adenoma or carcinoma, or extrapancreatic malignancy.
- Infants and Children: Leucine sensitivity, islet cell hyperplasia, nesidioblastosis, extrapancreatic malignancy, islet cell adenoma, or adenomatosis. Diazoxide Oral Suspension may be used preoperatively as a temporary measure, and postoperatively, if hypoglycemia persists.
Diazoxide should be used only after a diagnosis of hypoglycemia due to one of the above conditions has been definitely established. When other specific medical therapy or surgical management either has been unsuccessful or is not feasible, treatment with diazoxide should be considered.
Contraindications
Diazoxide is contraindicated in patients with:
- Functional hypoglycemia
- Hypersensitivity to diazoxide, any of the excipients in diazoxide, or other thiazides
Warnings
The antidiuretic property of diazoxide may lead to significant fluid retention. In patients with compromised cardiac reserve, fluid retention may precipitate congestive heart failure. If fluid retention develops, manage according to standards of care.
Co-administration of diazoxide with thiazides may potentiate the hyperglycemic and hyperuricemic actions of diazoxide (see DRUG INTERACTIONS and ANIMAL PHARMACOLOGY AND/OR TOXICOLOGY).
Ketoacidosis and nonketotic hyperosmolar coma have been reported in patients treated with diazoxide, usually during intercurrent illness. Prompt recognition and treatment are essential (see OVERDOSAGE), and prolonged surveillance following the acute episode is necessary because of the long drug half-life of approximately 30 hours. Advise patients to monitor urine glucose and ketones and to promptly report abnormal findings and symptoms of ketoacidosis to their healthcare provider. Transient cataracts occurred in association with hyperosmolar coma in an infant, and subsided on correction of the hyper-osmolarity. Cataracts have been observed in several animals receiving daily doses of intravenous or oral diazoxide.
The development of abnormal facial features in four children treated chronically (>4 years) with diazoxide for hypoglycemia hyperinsulinism in the same clinic has been reported.
Pulmonary Hypertension in Neonates and Infants
There have been postmarketing reports of pulmonary hypertension occurring in infants and neonates treated with diazoxide. The cases were reversible upon discontinuation of the drug. Monitor patients, especially those with risk factors for pulmonary hypertension, for respiratory distress and discontinue diazoxide if pulmonary hypertension is suspected.
Precautions
General:
Treatment with diazoxide should be initiated under close clinical supervision, with careful monitoring of blood glucose and clinical response until the patient's condition has stabilized. This usually requires several days. If not effective in 2 to 3 weeks, discontinue diazoxide.
Prolonged treatment requires regular monitoring of urine glucose and ketones, especially under stress conditions. Advise patients to promptly report any abnormalities to their healthcare provider. Periodically monitor blood glucose to determine the need for dosage adjustment.
Consider the effects of diazoxide on the hematopoietic system and the level of serum uric acid; the latter should be considered particularly in patients with hyperuricemia or a history of gout.
Since the plasma half-life of diazoxide is prolonged in patients with impaired renal function, a reduced dosage should be considered. Serum electrolyte levels should also be evaluated for such patients.
The antihypertensive effect of other drugs may be enhanced by diazoxide, and this should be kept in mind when administering it concomitantly with antihypertensive agents.
Because of the protein binding, administration of diazoxide with coumarin anticoagulants or its derivatives may require reduction in the dosage of the anticoagulant, although there has been no reported evidence of excessive anticoagulant effect. In addition, diazoxide may displace bilirubin from albumin; consider this when treating newborns with increased bilirubinemia.
Pulmonary hypertension has been reported in neonates and young infants treated with diazoxide (see WARNINGS).
Information for Patients:
Advise patients of the need for periodic laboratory testing during treatment with diazoxide. In addition, advise patients to:
- take diazoxide on a regular schedule as prescribed, not to skip doses, not to take extra doses
- consult their healthcare provider before starting any new medications
- not allow anyone else to take this medication
- follow dietary instructions
- report any adverse reactions (i.e., increased urinary frequency, increased thirst, fruity breath odor) promptly to their healthcare provider
- report pregnancy or to discuss plans for pregnancy with their healthcare provider
Laboratory tests:
Consider monitoring the following laboratory tests during treatment with diazoxide (not all-inclusive):
- blood glucose (recommended at periodic intervals in patients taking diazoxide orally for treatment of hypoglycemia, until stabilized)
- blood urea nitrogen (BUN) and creatinine clearance
- hematocrit, platelet count, total and differential leukocyte counts
- serum aspartate aminotransferase (AST)
- serum uric acid level
- urine testing for glucose and ketones
Drug Interactions:
Since diazoxide is highly bound to serum proteins, it may displace other substances which are also bound to protein, such as bilirubin or coumarin and its derivatives, resulting in higher blood levels of these substances. Concomitant administration of oral diazoxide and diphenylhydantoin may result in a loss of seizure control. Consider these potential interactions when administering diazoxide.
The concomitant administration of thiazides or other diuretics may potentiate the hyperglycemic and hyperuricemic effects of diazoxide.
Drug/Laboratory Test Interactions:
The hyperglycemic and hyperuricemic effects of diazoxide preclude proper assessment of these metabolic states. Increased renin secretion, IgG concentrations and decreased cortisol secretions have also been noted. Diazoxide inhibits glucagon-stimulated insulin release and causes a false-negative insulin response to glucagon.
Carcinogenesis, mutagenesis, impairment of fertility:
No long-term animal dosing study has been done to evaluate the carcinogenic potential of diazoxide. No laboratory study of mutagenic potential or animal study of effects on fertility has been done.
Reproduction studies using the oral preparation in rats have revealed increased fetal resorptions and delayed parturition, as well as fetal skeletal anomalies; evidence of skeletal and cardiac teratogenic effects in rabbits has been noted with intravenous administration. Diazoxide has also been demonstrated to cross the placental barrier in animals and to cause degeneration of the fetal pancreatic beta cells (see ANIMAL PHARMACOLOGY AND/OR TOXICOLOGY). Since there are no adequate data on fetal effects of this drug when given to pregnant women, safety in pregnancy has not been established. When the use of Diazoxide Oral Suspension is considered, the indications should be limited to those specified above for adults (see INDICATIONS AND USAGE), and the potential benefits to the mother must be weighed against possible harmful effects to the fetus.
Non-teratogenic effects:
Diazoxide crosses the placental barrier and appears in cord blood. When given to the mother prior to delivery of the infant, the drug may produce fetal or neonatal hyperbilirubinemia, thrombocytopenia, altered carbohydrate metabolism, and possibly other side effects that have occurred in adults.
Alopecia and hypertrichosis lanuginosa have occurred in infants whose mothers received oral diazoxide during the last 19 to 60 days of pregnancy.
Labor and delivery:
Since intravenous administration of the drug during labor may cause cessation of uterine contractions, and administration of oxytocic agents may be required to reinstate labor, caution is advised in administering diazoxide at that time.
Nursing mothers:
Information is not available concerning the passage of diazoxide in breast milk. Because many drugs are excreted in human milk and because of the potential for adverse reactions from diazoxide in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Adverse Reactions/Side Effects
Frequent and Serious:
Sodium and fluid retention is most common in young infants and in adults and may precipitate congestive heart failure in patients with compromised cardiac reserve (see DRUG INTERACTIONS).
Infrequent but Serious:
Diabetic ketoacidosis and hyperosmolar nonketotic coma may develop very rapidly. Monitor patients for up to 7 days due to the long half-life of diazoxide (see OVERDOSAGE).
Other frequent adverse reactions:
Hirsutism of the lanugo type, mainly on the forehead, back and limbs, occurs most commonly in children and women and may be cosmetically unacceptable. It subsides on discontinuation of the drug.
Hyperglycemia or glycosuria may require reduction in dosage in order to avoid progression to ketoacidosis or hyperosmolar coma.
Gastrointestinal intolerance may include anorexia, nausea, vomiting, abdominal pain, ileus, diarrhea, transient loss of taste.
Tachycardia, palpitations, increased levels of serum uric acid are common.
Thrombocytopenia with or without purpura may require discontinuation of the drug. Neutropenia is transient, is not associated with increased susceptibility to infection, and ordinarily does not require discontinuation of the drug. Skin rash, headache, weakness, and malaise may also occur.
Other adverse reactions:
Cardiovascular: Hypotension occurs occasionally, which may be augmented by thiazide diuretics given concurrently. A few cases of transient hypertension, for which no explanation is apparent, have been noted. Chest pain has been reported rarely. Pulmonary hypertension has been reported in neonates and young infants (see WARNINGS).
There have been postmarketing reports of pericardial effusion in patients without structural heart disease; the majority of cases occurred in pediatric patients and infants.
Gastrointestinal: There have been postmarketing reports of necrotizing enterocolitis; the majority of cases occurred in infants with underlying co-morbid conditions.
Hematologic: eosinophilia; decreased hemoglobin / hematocrit; excessive bleeding, decreased IgG.
Hepato-renal: increased AST, alkaline phosphatase; azotemia, decreased creatinine clearance, reversible nephrotic syndrome, decreased urinary output, hematuria, albuminuria. Neurologic: anxiety, dizziness, insomnia, polyneuritis, paresthesia, pruritus, extrapyramidal signs.
Ophthalmologic: transient cataracts, subconjunctival hemorrhage, ring scotoma, blurred vision, diplopia, lacrimation. Skeletal, integumentary; monilial dermatitis, herpes, advance in bone age; loss of scalp hair. Systemic: fever, lymphadenopathy. Other; gout acute pancreatitis/pancreatic necrosis, galactorrhea, enlargement of lump in breast.
Related/similar drugs
Overdosage
An overdosage of diazoxide causes marked hyperglycemia which may be associated with ketoacidosis. It will respond to prompt insulin administration and restoration of fluid and electrolyte balance. Because of the drug's long half-life (approximately 30 hours), the symptoms of overdosage require prolonged surveillance for periods up to seven days until the blood glucose level stabilizes within the normal range. One investigator reported successful lowering of diazoxide blood levels by peritoneal dialysis in one patient and by hemodialysis in another.
Diazoxide Dosage and Administration
Patients should be under close clinical observation when treatment with diazoxide is initiated. Carefully monitor the clinical response and blood glucose until the patient’s condition has stabilized satisfactory; in most instances, this may be accomplished in several days. If administration of diazoxide is not effective after 2 or 3 weeks, discontinue diazoxide.
Individualize the dosage of diazoxide based on the severity of the hypoglycemic condition and the blood glucose level and clinical response of the patient. Adjust the dosage until the desired clinical and laboratory effects are produced with the least amount of diazoxide. Take special care to ensure the accuracy of the dosage in infants and young children.
ANIMAL PHARMACOLOGY AND/OR TOXICOLOGY
Oral diazoxide in the mouse, rat, rabbit, dog, pig, and monkey produces a rapid and transient rise in blood glucose levels. In dogs, increased blood glucose is accompanied by increased free fatty acids, lactate, and pyruvate in the serum. In mice, a marked decrease in liver glycogen and an increase in the blood urea nitrogen level occur.
In acute toxicity studies the LD50 for orally administered diazoxide is >5,000 mg/kg in the rat, >522 mg/kg in the neonatal rat, between 1,900 and 2,572 mg/kg in the mouse, and 219 mg/kg in the guinea pig. Although the oral LD50 was not determined in the dog, a dosage of up to 500 mg/kg was well tolerated.
In subacute oral toxicity studies, diazoxide at 400 mg/kg in the rat produced growth retardation, edema, increases in liver and kidney weights, and adrenal hypertrophy. Daily dosages up to 1,080 mg/kg for three months produced hyperglycemia, an increase in liver weight and an increase in mortality. In dogs given oral diazoxide at approximately 40 mg/kg/day for one month, no biologically significant gross or microscopic abnormalities were observed. Cataracts, attributed to markedly disturbed carbohydrate metabolism, have been observed in a few dogs given repeated daily doses of oral or intravenous diazoxide. The lenticular changes resembled those which occur experimentally in animals with increased blood glucose levels. In chronic toxicity studies, rats given a daily dose of 200 mg/kg diazoxide for 52 weeks had a decrease in weight gain and an increase in heart, liver, adrenal and thyroid weights. Mortality in drug-treated and control groups was not different. Dogs treated with diazoxide at dosages of 50, 100, and 200 mg/kg/day for 82 weeks had higher blood glucose levels than controls. Mild bone marrow stimulation and increased pancreas weights were evident in the drug-treated dogs; several developed inguinal hernias, one had a testicular seminoma, and another had a mass near the penis. Two females had inguinal mammary swellings. The etiology of these changes was not established. There was no difference in mortality between drug-treated and control groups. In a second chronic oral toxicity study, dogs given milled diazoxide at 50, 100, and 200 mg/kg/day had anorexia and severe weight loss, causing death in a few. Hematologic, biochemical, and histologic examination did not indicate any cause of death other than inanition. After one year of treatment, there is no evidence of herniation or tissue swelling in any of the dogs.
When diazoxide was administered at high dosages concomitantly with either chlorothiazide to rats or trichlormethiazide to dogs, increased toxicity was observed. In rats, the combination was nephrotoxic; epithelial hyperplasia was observed in the collecting tubules. In dogs, a diabetic syndrome was produced which resulted in ketosis and death. Neither of the drugs given alone produced these effects.
Although the data are inconclusive, reproduction and teratology studies in several species of animals indicate that diazoxide, when administered during the critical period of embryo formation, may interfere with normal fetal development, possibly through altered glucose metabolism. Parturition was occasionally prolonged in animals treated at term. Intravenous administration of diazoxide to pregnant sheep, goats, and swine produced in the fetus an appreciable increase in blood glucose level and degeneration of the beta cells of the Islets of Langerhans. The reversibility of these effects was not studied.
How is Diazoxide supplied
Diazoxide Oral Suspension, USP 50 mg/mL, a chocolate-mint flavored suspension; bottle of 30 mL (NDC 13517-100-30), with dropper calibrated to deliver 10, 20, 30, 40 and 50 mg diazoxide.
Shake well before each use. Protect from light. Store in carton until contents are used. Store in light resistant container as defined in the USP. Store Diazoxide Oral Suspension at 25°C (77°F) excursions permitted 15°-30°C (59-86°F). [See USP Controlled Room Temperature].
Rx only
Manufactured for:
e5 Pharma, LLC
Boca Raton, FL 33432
1000001287 Rev. 01/2025
| DIAZOXIDE
diazoxide suspension |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - e5 Pharma, LLC (933360179) |
Frequently asked questions
- Should you use a diuretic with diazoxide?
- How does diazoxide (Proglycem) cause hyperglycemia?
- Is diazoxide a vasodilator?
- How does diazoxide slow insulin?
More about diazoxide
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (1)
- Side effects
- Dosage information
- Patient tips
- During pregnancy
- Drug class: agents for hypertensive emergencies
- Breastfeeding
- En español

